Question
Question: The acid in which O-O bonding is present is: A.\[{H_2}{S_2}{O_3}\] B.\[{H_2}{S_2}{O_6}\] C.\[{...
The acid in which O-O bonding is present is:
A.H2S2O3
B.H2S2O6
C.H2S2O8
D.H2S4O6
Solution
Hint: An O-O linkage is known as a peroxy linkage. The compounds which have an -O-O-H atomic group in place of an -O-H group are peroxy acids. They are also known as peracids.
Complete step by step answer:
Peroxy acids are the class of chemical compounds in which the -OH group is replaced by the atomic group -OOH. Peroxy acids usually are prepared by reaction of the oxy acid with hydrogen peroxide; small amounts of sulfuric or other strong acids often are used to accelerate the reaction of weak oxy acids. The peroxy acids are used primarily as oxidizing agents; they readily add oxygen to alkenes to give epoxides and are used to convert ketones to esters and amines to nitro compounds, amine oxides, or nitroso compounds.
The general formula of a peroxy acid is R-O-O-R’.
Carboxylic acids are better acids than peroxy acids because the carboxylate ion is stabilised by resonance between the two oxygens which is not the case in the conjugate base of peroxy acids.
Now, these oxyacids have special structures.
The structure of H2S2O3 is:
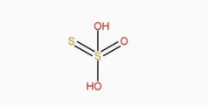
The structure of H2S2O6 is:
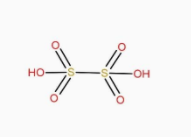
The structure of H2S2O8 is:
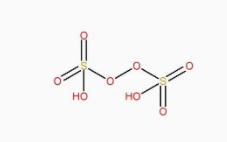
The structure of H2S4O6 is:
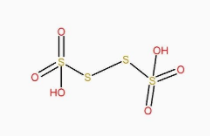
It can be clearly seen that H2S2O8 has peroxy linkage in it.
So, H2S2O8 is a peroxy acid.
Hence, the correct answer is (C)__
Note: These oxyacids have special structures. So, one must remember these structures to answer this question correctly.
