Question
Question: The absorption maxima of some complex ions are as follows: \({{\left[ CrC{{l}_{6}} \right]}^{3-}}-...
The absorption maxima of some complex ions are as follows:
[CrCl6]3−−760 nm
[Cr(NH3)6]3+−470 nm
[Cr(H2O)6]3+−695 nm
The order of Δofor these ions is
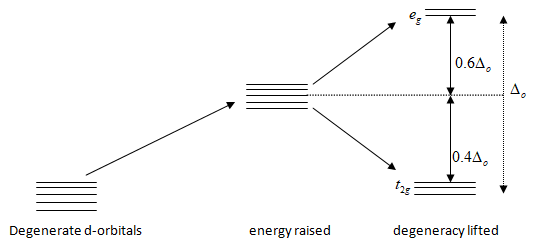
a.) [Cr(NH3)6]3+[Cr(H2O)6]3+>[CrCl6]3−
b.) [CrCl6]3−[Cr(H2O)6]3+>[Cr(NH3)6]3+
c.) [Cr(H2O)6]3+[Cr(NH3)6]3+>[CrCl6]3−
d.) [CrCl6]3−[Cr(NH3)6]3+[Cr(H2O)6]3+
Solution
Δo Depends on the crystal field splitting, the lesser is the value of wavelength of light the maximum is the crystal field splitting. Therefore using the above given wavelengths find out the correct order of Δo for the ions. Also we can find the answer with the order of splitting of the ions either if geometry and the ligands are held constant or geometry and the metal are held constant by taking them as a reference.
Complete Solution :
Given that [CrCl6]3−−760 nm
[Cr(NH3)6]3+−470 nm
[Cr(H2O)6]3+−695 nm
For the above given ions the order of the wave length is
[Cr(NH3)6]3+<[Cr(H2O)6]3+<[CrCl6]3−
We know that Δo depends on the crystal field splitting.
The lesser is the value of wavelength of light the maximum is the crystal field splitting.
Therefore the order of the Δo for these ions is
[Cr(NH3)6]3+[Cr(H2O)6]3+>[CrCl6]3−
Also we can have the same answer by taking the below order of the ions as the reference since the geometry and the metal are held constant.
The splitting of d orbitals in the CF model not only depends on the geometry of the complex, it also depends on the nature of the metal ion, the charge on this ion and the ligands that surround this ion.
When the geometry and the ligands are held constant, this splitting decreases in the following order: Pt4+>Ir3+>Rh3+>Co3+>Cr3+>Fe3+>Fe2+>Co2+>Ni2+>Mn2+
When the geometry and the metal are held constant, the splitting of the d- orbitals increases in the following order:
I−<Br−<[NCS]−<Cl−<F−<OH−<H2O<NH3<en<CN−<CO
Therefore the correct order of Δo for the above given ions is
[Cr(NH3)6]3+[Cr(H2O)6]3+>[CrCl6]3−
So, the correct answer is “Option (a)”.
Note: Δo is nothing but the difference between eg and t2g energy levels. The net energy of t2g with some x electrons andeg with y configuration relative to the center of the energy level is called the ligand field stabilization energy which is represented as LSFE.
