Question
Question: The absolute configurations of the centres (1 and 2) in the molecule given below are ...
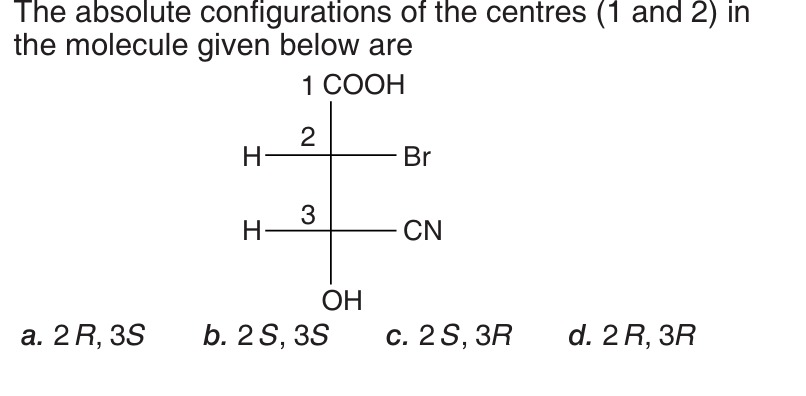
The absolute configurations of the centres (1 and 2) in the molecule given below are
2 R, 3S
2 S, 3S
2 S, 3R
2 R, 3R
2 R, 3S
Solution
The absolute configurations of the chiral centers are determined using the Cahn-Ingold-Prelog (CIP) priority rules and the R/S convention. In a Fischer projection, the horizontal lines represent bonds coming out of the plane, and the vertical lines represent bonds going into the plane. The lowest priority group (usually H) should be on a vertical line for the determined configuration to be correct. If the lowest priority group is on a horizontal line, the determined configuration is the opposite of the actual configuration.
For chiral center 2: The groups attached to carbon 2 are H, Br, COOH, and the rest of the chain (from carbon 3 downwards). Priority assignment:
- Br (atomic number 35)
- COOH (C bonded to O, O, O)
- The rest of the chain starting from carbon 3 (C bonded to H, C(of CN), O(of OH))
- H (atomic number 1)
Comparing COOH and the rest of the chain: COOH: C bonded to O, O, O. Rest of the chain: C bonded to H, C, O. Comparing the atoms attached to the first carbon after the chiral center: O (Z=8) vs O (Z=8). Next, O (Z=8) vs C (Z=6). So, COOH has higher priority than the rest of the chain. Priority order for carbon 2: Br (1) > COOH (2) > Rest of chain (3) > H (4). In the Fischer projection, H is on a horizontal line. Tracing 1->2->3: Br (right) -> COOH (top) -> Rest of chain (bottom). This is counterclockwise. Since H is on a horizontal line, the actual configuration is the opposite of counterclockwise, which is R. So, the configuration at carbon 2 is R.
For chiral center 3: The groups attached to carbon 3 are H, CN, OH, and the upper part of the chain (from carbon 2 upwards). Priority assignment:
- OH (O bonded to H)
- Upper part of the chain (C bonded to H, Br, COOH)
- CN (C bonded to N, N, N)
- H
Comparing the upper part of the chain and CN: Upper part: C (carbon 2) bonded to H, Br, C (of COOH). CN: C bonded to N, N, N. Comparing the atoms attached to the first carbon after the chiral center: Br (Z=35) vs N (Z=7). Br has a higher atomic number. So, the upper part of the chain has higher priority than CN. Priority order for carbon 3: OH (1) > Upper part of chain (2) > CN (3) > H (4). In the Fischer projection, H is on a horizontal line. Tracing 1->2->3: OH (bottom) -> Upper part of chain (top) -> CN (right). This is clockwise. Since H is on a horizontal line, the actual configuration is the opposite of clockwise, which is S. So, the configuration at carbon 3 is S.
The absolute configurations of the centers (2 and 3) are 2R and 3S.
