Question
Question: The absolute configuration of given compound is: 
A: (2S, 3R)
B: (2S, 3S)
C: (2R, 3R)
D: (2R, 3S)
Solution
An absolute configuration of a compound is the spatial arrangement of the atoms in a chiral molecule and its stereochemical description is termed as R or S, which refers to Rectus and Sinister, respectively.
Complete answer
If you rotate the curve in the clockwise manner, the absolute configuration is termed as R (rectus) configuration. On the other hand, the absolute configuration is termed as S (sinister) configuration if the curve is rotated in counter-clockwise direction. The lowest priority functional group should be located on the vertical line. Otherwise, the absolute configuration is reversed. The order of priority for functional group in the decreasing order is given as follows:
carboxylic acid > sulphonic acid > esters > amide > nitrile > aldehydes > ketones > alcohol > amines > hydrocarbons > ethers > alkyl halides
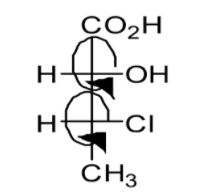
Referring to the above image, it is clear that the absolute configuration of the given compound is 2R, 3R.
Therefore the correct option is C. 2R,3R
Note:
It should be noted down that D-L system labelling is used in case of the whole molecule. On the other hand, R/S system labelling is used in case of the absolute configuration of each chiral carbon (chirality centre). D-L system doesn't have a direct connection with the (+)/(-) notation. While, (D-) and (L-) is exactly the same as (+) and (-) notation. In case of clockwise direction, the rotation is positive ("+") called dextrorotatory (D). In case of counter-clockwise direction, the rotation is negative ("-") and called as levorotatory (L).
