Question
Question: The above shown polymer is obtained when a carbon compound is allowed to stand. It is a white solid....
The above shown polymer is obtained when a carbon compound is allowed to stand. It is a white solid. The polymer is:
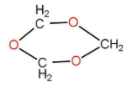
A.Trioxane
B.Formose
C.Paraformaldehyde
D.Metaldehyde
Solution
The polymer contains three oxygen groups and it is used with formaldehyde and paraformaldehyde. It is also used in the production of polyoxymethylene plastics.
Complete step by step answer:
1,3,5 trioxane is also known as trioxane, is a compound with the structure as follow:
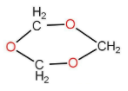
It has a chloroform like smell and is a white solid. It exists in three isomers. Basically the formation of trioxane is done by acid catalysed cyclic trimerization of formaldehyde with concentrated sulfuric acid. Three moles of formaldehyde form trioxane. The water molecule reacted and generated back with the product. So basically a carbon compound that is formaldehyde is getting converted to tricyclic compound. The reaction occurs as follow:
3H2CO+3H2O⇌C3H6O3+3H2O
Hence, the correct option is A.
Additional Information:
Formose is a naming reaction that was discovered by Aleksandr butlerov. Paraformaldehyde is produced by the polymerization of formaldehyde. Metaldehyde is an organic compound with the molecular formula CH3CHO4. It is used as a pesticide and it is a cyclic trimer of acetaldehyde.
Note:
Formaldehyde is a simple chemical compound made of hydrogen and oxygen. It is the simplest aldehyde. In pure state it occurs as a gas and is pungent smelling and colourless gas. The formaldehyde gas polymerises into paraformaldehyde gas readily. It is used for the synthesis of many complex compounds including urea formaldehyde resin, phenol formaldehyde resin, diphenyl isocyanide. It is also used as disinfectant as it kills many bacteria and fungi, when it is in aqueous solution.
