Question
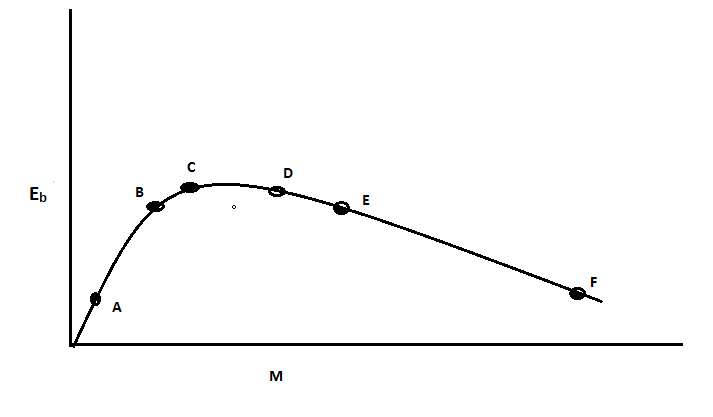
Question: The above is a plot of binding energy per nucleon \({E_b}\) , against the nuclear mass M; A, B, C, D...
The above is a plot of binding energy per nucleon Eb , against the nuclear mass M; A, B, C, D, E, F correspond to different nuclei. Consider four reactions:
(i).A+B→C+ε
(ii).C→A+B+ε
(iii).D+E→F+ε
(iv).F→D+E+ε
Where ε is the energy released? In which reaction is ε positive?
A.(i) and (iv)
B.(i) and (iii)
C.(ii) and (iv)
D.(ii) and (iii)
Solution
In the figure, we have to analyze the positions of different elements and compare their nuclear masses. Also, we have to keep in mind the concept of binding energy and mass defect to reach our solution. When a nuclear reaction takes place, either energy is absorbed or released. Keeping this in mind, we can theoretically reach our solution.
Complete Step By Step answer:
From the analysis of the figure, we can deduce that elements A, B and C have light-weighted nucleus whereas D, E and F have a heavy nucleus. Also, the binding energy of B, C and D elements are higher than elements A, E and F. Now, as we know, the binding energy is the minimum energy required to break an atom’s nucleus into its constituent particles that is protons and neutrons. This means that the higher the binding energy of an atom, the more energy is required to break the nucleus. The binding energy per nucleon is higher for stable elements. Now, here comes the concept of mass defect which states that the total mass of the atomic nucleus is less than the mass of protons and neutrons in the nucleus. This mass defect accounts for the energy released in a reaction.
Whenever a reaction takes place, it always wants to achieve a stable state. To achieve a stable state, it has to release an amount of energy. Now, we will compare the binding energy of each reaction to find the most stable reactions.
In reaction (i), the binding energy of A and B is less than C which implies that the reaction is more stable as the binding energy of the product is more than the binding energy of the reactants and hence the energy released is positive. Here, we can see that two lighter nuclei particles get fused to produce a heavy particle. This process is called fusion.
In the second and third reactions, we observe that the binding energy of the product is less than the binding energy of the reactants and hence it’s less stable. An amount of energy is absorbed in these reactions.
If we observe the fourth reaction, we will notice that, As F has heavy nuclei, it undergoes fission to achieve stability and produces D and E and an amount of energy is released. Also, the binding energy of D and E combined is greater than the reactant F.
Therefore, option A is the correct answer.
Note:
Whether energy is positive or negative is determined by the Q-value of the reaction. For a reaction, Q-value refers to the amount of energy absorbed or released during a reaction. It can be determined if the masses of the reactants and the products are known. The formula of Q-value is given by:
⇒Q=(mr−mp)×0.9315Gev
Where,
Q is the Q-value.
mr is the mass of reactants.
mp is the mass of products.
If the Q-value is positive, then it’s an exothermic reaction i.e. energy is released during the reaction and if the Q-value is negative then it’s an endothermic reaction i.e. energy is absorbed during the reaction.
