Question
Question: The \[0.2\] moles of an ideal gas is taken around the cycle abc as shown in the figure. The path b-c...
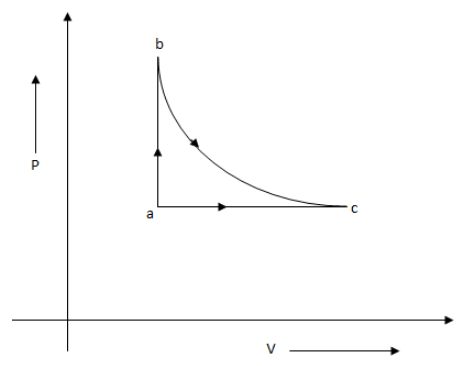
The 0.2 moles of an ideal gas is taken around the cycle abc as shown in the figure. The path b-c is adiabatic process, a-b is isovolumic process and c-a is isobaric process. The temperature at a and b are Ta=300K and Tb=500K and pressure at a is 1 atmosphere. Find the volume at c.
( Given γ=CvCp=35 , R=8.205×10−2L atm mol−1K−1 )
A. 6.9 L
B. 6.68 L
C. 5.52 L
D. 5.82 L
Solution
We are asked to find the volume at point c. To find the volume at point c, we need to find the pressure and volume at point b. Here, you will need to use Gay-Lussac’s law to find the pressure at point b and use ideal gas equation to find the volume at point b. Then use the equation for the adiabatic process to find the volume at point c.
Complete step by step answer:
Given, number of moles, n=0.2.
The path b-c is adiabatic process, a-b is isovolumic process and a-c is isobaric process.
The temperature at point a, Ta=300K.
The temperature at point b, Tb=500K.
Pressure at point a, Pa=1 atm.
Ratio of specific heats, γ=CvCp=35.
Gas constant, R=8.205×10−2L atm mol−1K−1.
Let the pressure at point b and c be Pb and Pc respectively.
Let the volume at point b and c be Vb and Vc respectively.
The path a-b is an isovolumic process, which means the volume is constant for path a-b.
According to Gay-Lussac’s law when volume is constant for an ideal gas, we can write
TP=constant
Applying Gay-Lussac’s law for path a-b we get,
TaPa=TbPb
Putting the values of Pa, Ta and Tb we get,
3001=500Pb
⇒500Pb=3001
⇒Pb=35atm
From ideal gas equation we have,
PV=nRT
where P is the pressure, V is the volume, n is the number of moles, R is gas constant and T is temperature.
Using ideal gas equation at point b we get,
PbVb=nRTb
Putting the values of Pb, n, R and Tb we get,
35Vb=2×8.205×10−2×500
⇒Vb=53×2×8.205×10−2×500
⇒Vb=4.92L
The path b-c is an adiabatic process.
For adiabatic process we have the equation,
PVγ=constant
where P is the pressure, V is the volume and γ is ratio of specific heats.
Using this equation for path b-c we get,
PbVbγ=PcVcγ (i)
From the graph, we get Pc=Pa=1atm.
Now, putting the values of Pb, Vb, Pc and γ in equation (i) we get,
35×4.9235=1×Vc35
⇒Vc=35×4.923553
⇒Vc=3553×4.92
∴Vc=6.68L
Therefore, the volume at c is 6.68L
Hence, the correct answer is option B.
Note: There are four gas laws which are important to remember, these are
1. Boyle’s law:- Boyle’s law states that when temperature is constant, then pressure of the gas is inversely proportional to its volume.
2. Charles’s law:- Charles’ law states that volume occupied by a gas is proportional to the temperature of the gas at constant pressure.
3. Avogadro’s law:- Avogadro’s law states that different gases of equal volume contain equal numbers of moles at same temperature and pressure.
4. Gay-Lussac’s law:- Gay-Lussac’s law states that for constant volume, pressure is directly proportional to the temperature of the gas.
