Question
Question: The \[0--0--H\] bond angle in \[{H_2}{0_2}\] is: A. \[106^\circ \;\] B. \[109^\circ 28'\] C...
The 0−−0−−H bond angle in H202 is:
A. 106∘
B. 109∘28′
C. 120∘
D. 94∘
Solution
Oxygen molecule has a valency of -2. That is, it can form two sigma bonds with the other atoms. It has three lone pairs to form pi bonds as well with other atoms. H202 has all two oxygen atoms and it’s a nonplanar molecule with sp3 hybridization.
Complete step by step answer:
The Lewis structure for hydrogen peroxide H202 consists of an oxygen atom with a single bond to another oxygen atom and each oxygen atom has two single bonds with one-two hydrogen atoms. Lewis structure is based on the octet rule which states that there should be eight electrons in the outermost shell or orbit of an atom for the molecule to be stable.
There are six valence electrons for each molecule of Oxygen in H202 and thus the total number of valence electrons is 6×3=18 . As the octet rule applies, the hydrogens have 1 valence electron in its valence shell.
The oxygen atoms have two lone pairs of electrons which are making it stable due to the eight electrons in its outermost orbit. To satisfy the octet rule, oxygen atoms require to form a single bond with another oxygen atom. As both the atoms of Oxygen on sides have the same electronegativity and structure, the single bond electrons keep in the middle of the single oxygen -oxygen bond.
Here, the lone pairs of electrons on the oxygen atoms repel the electrons in the two side bonds present, forcing the atom to adopt a bent molecular geometry. Expected geometry for each oxygen is tetrahedral, where the O−O−H bond angle to be 94o . However, as per the context of the VSEPR model, lone pairs of electrons are considered slightly more repulsive than bonding pairs of electrons due to their closer proximity to the central atom.
The structure of hydrogen peroxide is non-planar and looks like an open book. In this structure, the bond angle of the O−O−H bond is around 98o . The structure is shown below,
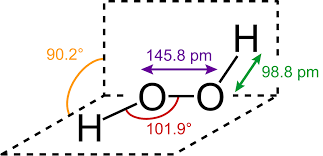
So, the correct answer is D.
Note: Ozone can exist in two forms, one is syn other one is anti. In the case of syn, there is a dipole moment and in the case of anti, it has zero dipole moment. This is because in anti the lone pairs of oxygen atoms are in opposite directions therefore it nullifies each dipole and the resultant becomes zero. But in the case of Syn form, the lone pairs dipoles are in the same direction, so the overall dipole is additive in nature.
