Question
Question: $\text{Ph-NH-SO}_3\text{Ph} \xrightarrow[]{\text{OH}^{\ominus}\text{/H}_2\text{O}}$ product of given...
Ph-NH-SO3PhOH⊖/H2O product of given reaction is
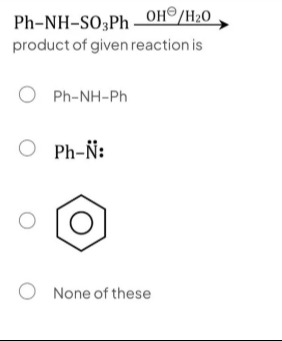
A
Ph-NH-Ph
B
Ph-N¨:
C
None of these
Answer
Ph–NH–Ph
Explanation
Solution
The sulfonamide (Ph–NH–SO₃Ph) undergoes base‐mediated hydrolysis. In the presence of OH⁻ (in H₂O) the S–N bond is cleaved. As a result, the sulfonyl group is removed (as a sulfonate ion) and the free amine (diphenylamine, Ph–NH–Ph) is formed.
Minimal Explanation:
-
OH⁻ attacks the sulfonamide and cleaves the S–N bond.
-
The sulfonyl group leaves as a sulfonate ion.
-
The resulting product is diphenylamine (Ph–NH–Ph).
