Question
Question: $\text{Equivalent wt. =} \frac{\text{atomic wt}}{\text{Valency}} \text{OR} \frac{\text{Mol.w.t}}{\te...
Equivalent wt. =Valencyatomic wtORValencyMol.w.t
Calculate n-f (Both Sides)
① 2Cl−→Cl2
② Fe2O3→FeO
③ N3−→NH3
④ Fe2O3→Fe0.95O
⑤ Fe3O4→FeO
⑥ H3PO2→PH3
⑦ SO2→H2S
⑧ SO3→H2SO4
⑨ C2O42−→CO2
⑩ H2O2→H2O
⑪ H2O2→O2
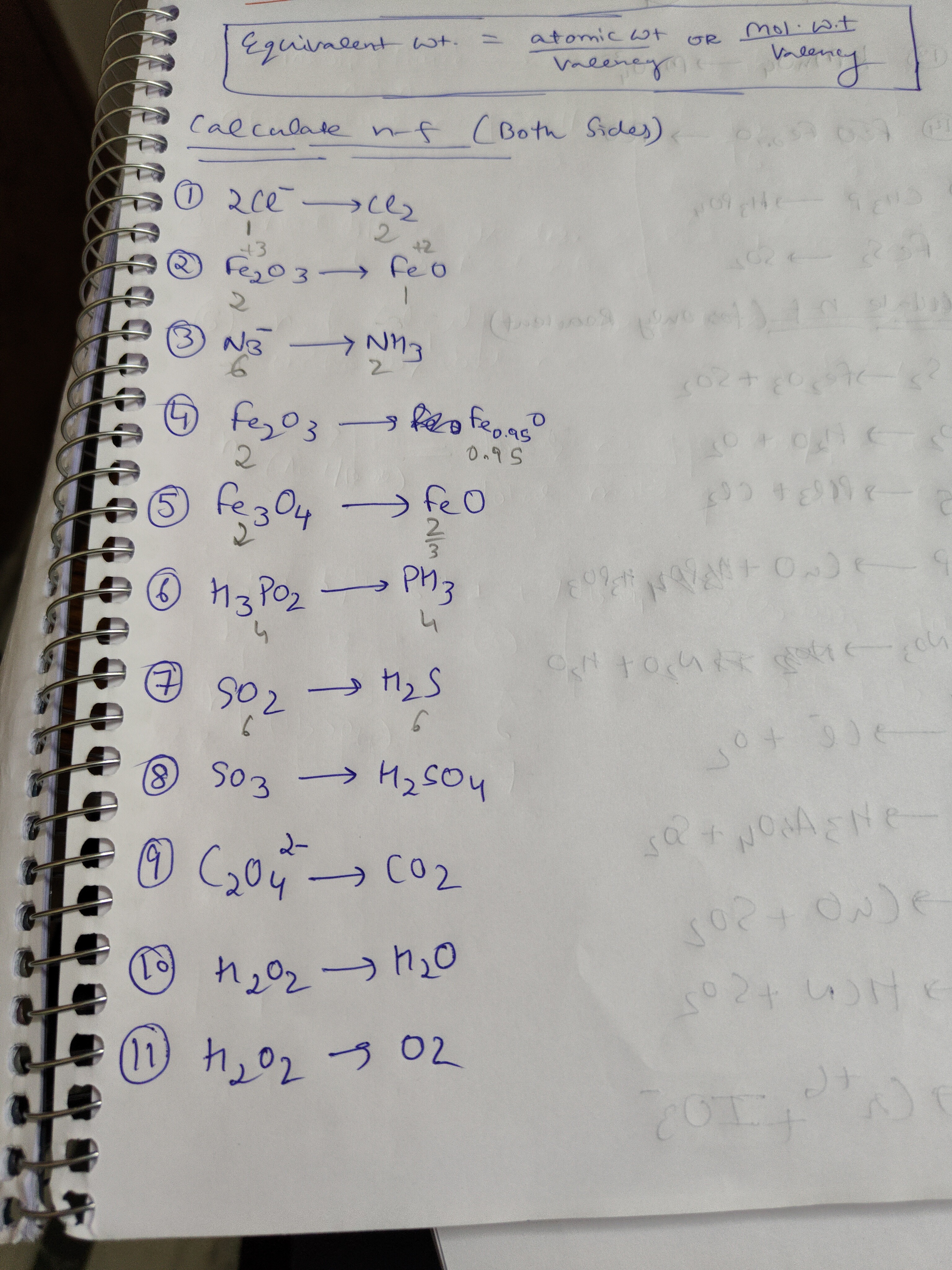
The n-factors (reactant, product) are as follows: ① 2Cl−→Cl2: (1, 2) ② Fe2O3→FeO: (2, 1) ③ N3−→NH3: (8, 8/3) ④ Fe2O3→Fe0.95O: (34/19, 17/20) ⑤ Fe3O4→FeO: (2, 2/3) ⑥ H3PO2→PH3: (4, 4) ⑦ SO2→H2S: (6, 6) ⑧ SO3→H2SO4: (2, 2) ⑨ C2O42−→CO2: (2, 1) ⑩ H2O2→H2O: (2, 1) ⑪ H2O2→O2: (2, 2)
Solution
The n-factor for each substance is calculated based on the change in oxidation state of the element undergoing redox. For the reactant, we determine the change in oxidation state from its initial state to its final state in the product, multiplied by the number of atoms of that element in the reactant molecule/ion. For the product, we consider the reverse reaction and calculate its n-factor similarly. For non-redox reactions (like SO3→H2SO4), the n-factor is determined by its acid-base properties (basicity/acidity).
