Question
Question: $\text{CH}_3\text{CHO}$ + $\overset{\text{(iv) C}_2\text{H}_6\text{OH}}{\leftarrow}$ $\text{N}_2^+...
CH3CHO + ←(iv) C2H6OH N2+Cl−
Br
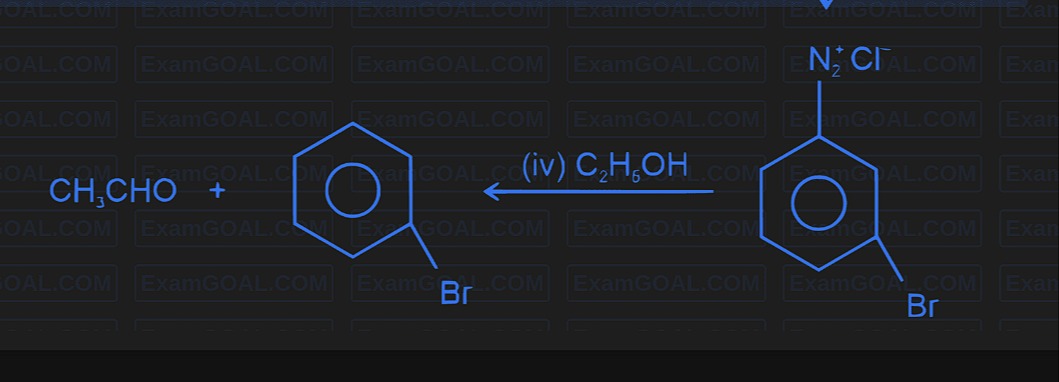
The final product is bromobenzene, produced via the Sandmeyer reaction.
Solution
A key step in many aromatic substitution syntheses is the conversion of an arenediazonium salt into a halo‐substituted arene via the Sandmeyer reaction. In the scheme shown the benzene–diazonium chloride (–N₂⁺Cl⁻, prepared earlier from an aniline derivative) is converted to bromobenzene by treatment with a “Br⁻” source (usually in the presence of CuBr as catalyst). (The preceding steps – for example, the use of CH₃CHO and the ethanolic reagent – are part of the overall synthesis but the final substitution step is the Sandmeyer reaction.)
Thus, the reaction depicted at the end:
Ar–N2+Cl−CuBrAr–Br+N2↑identifies the conversion of the diazonium salt to bromobenzene.
Core Explanation:
-
A diazonium salt (Ar–N₂⁺Cl⁻) is prepared (from an aniline derivative, not shown).
-
On treatment with CuBr (or a bromide source), the diazonium group is replaced by Br via the Sandmeyer reaction.
-
Hence, bromobenzene is obtained.
