Question
Question: CH3 CH3-CH+H₂C=CH2 CH3...
CH3
CH3-CH+H₂C=CH2
CH3
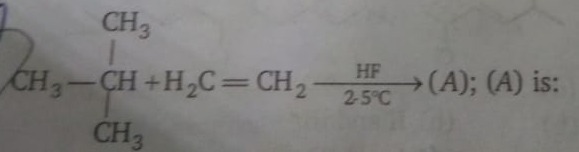
Answer
The product (A) is 2‑fluoro‑2,2‑dimethylbutane.
Explanation
Solution
Explanation:
- Carbocation formation: Reaction of 2‐methylpropane (isobutane) with HF (a strong acid) leads (via hydride abstraction) to formation of the tertiary (tert‐butyl) carbocation, (CH₃)₃C⁺.
- Alkylation: The ethene (H₂C=CH₂) reacts with the tert‐butyl cation to give a new carbocation, namely, CH₃–C⁺(CH₃)₂–CH₂CH₃.
- Fluoride attack: Finally, fluoride ion (F⁻) attacks this carbocation to yield the alkyl fluoride product:
CH₃–C(F)(CH₃)₂–CH₂CH₃.
