Question
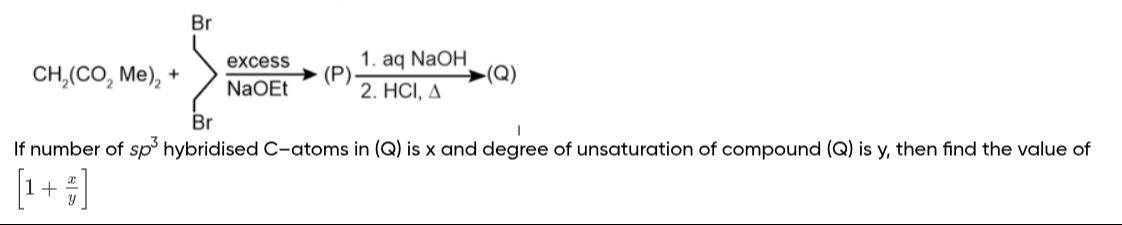
Question: CH$_2$(CO$_2$Me)$_2$ + $\xrightarrow[\text{NaOEt}]{\text{excess}}$ (P) $\xrightarrow[2. \text{ HCl, ...
CH2(CO2Me)2 + excessNaOEt (P) 1. aq NaOH2. HCl, Δ (Q)
If number of sp3 hybridised C-atoms in (Q) is x and degree of unsaturation of compound (Q) is y, then find the value of [1+yx]
Answer
3
Explanation
Solution
The reaction sequence leads to cyclobutanecarboxylic acid (Q). The cyclobutane ring contains 4 sp3 hybridized carbon atoms, so x=4. The molecule has one ring and one carbonyl group, giving a degree of unsaturation y=2. Therefore, [1+yx]=[1+24]=3.
