Question
Question: $\text{CH-CH}_3$ $\text{H}_3\text{C-CH=CH-C=CH-CH=CH-CH}_3$...
CH-CH3 H3C-CH=CH-C=CH-CH=CH-CH3
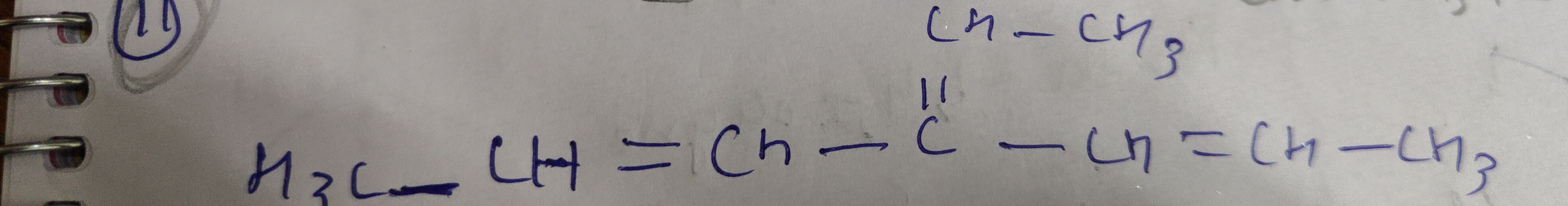
Answer
Octa-2,6-diene-4-yne
Explanation
Solution
The given condensed structure H3C-CH=CH-C=CH-CH=CH-CH3 is interpreted as CH3-CH=CH-C≡C-CH=CH-CH3 because a literal interpretation of C=CH would lead to an impossible valency for carbon. The longest chain containing all multiple bonds is 8 carbons (octa). There are two double bonds at positions 2 and 6 (diene) and one triple bond at position 4 (yne). Numbering from either end gives the same set of locants (2,4,6) for the multiple bonds. The name is formed by combining the parent alkane name with the suffixes for double and triple bonds, ensuring the 'e' from 'ene' is dropped before 'yne'.
