Question
Question: $\text{286. }CH_2=C=CH_2 \xrightarrow{\text{HCl (excess)}}$...
286. CH2=C=CH2HCl (excess)
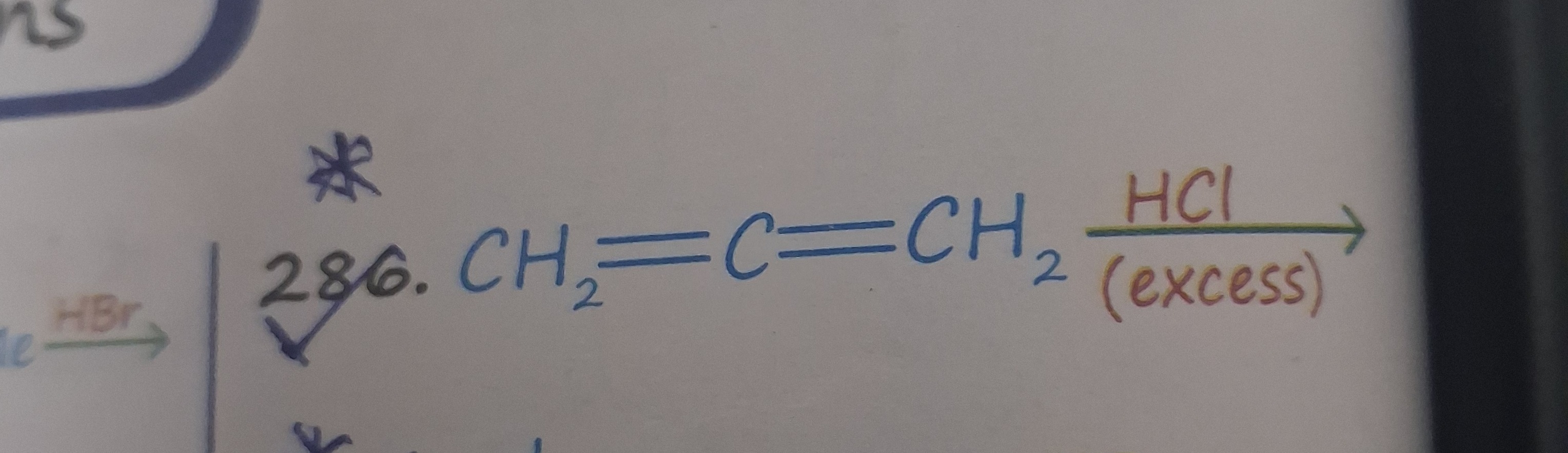
A
2-chloropropene
B
2,2-dichloropropane
C
1,2-dichloropropane
D
1,1-dichloropropane
Answer
2,2-dichloropropane
Explanation
Solution
The reaction involves the electrophilic addition of excess HCl to allene (CH2=C=CH2).
The first addition of HCl to allene yields 2-chloropropene (CH3−C(Cl)=CH2) via a resonance-stabilized vinylic carbocation intermediate.
The second addition of excess HCl to 2-chloropropene follows Markovnikov's rule. The proton adds to the terminal carbon (CH2) to form a more stable tertiary carbocation (CH3−C(Cl)−C+H3). The chloride ion then attacks this carbocation, resulting in the formation of 2,2-dichloropropane (CH3−C(Cl)(Cl)−CH3).
