Question
Question: \({\text{S}}{{\text{O}}_{\text{3}}}\) is an electrophile in the sulphonation of benzene. A) True ...
SO3 is an electrophile in the sulphonation of benzene.
A) True
B) False
Solution
We know that sulphonation of benzene means reaction of benzene with a mixture of sulphur trioxide and sulphuric acid. When benzene reacts with a mixture of sulphur trioxide and sulphuric acid benzene sulphonic acid is formed.
Complete answer: We know that benzene is a planar molecule and has a cloud of delocalised electrons above the plane of the ring. Thus, benzene is rich in electrons. As a result, benzene is highly attractive towards the species that are electron deficient i.e. electrophiles.
-During sulphonation of benzene, benzene is heated with a mixture of sulphur trioxide and sulphuric acid. In the reaction, benzene sulphonic acid is produced. Sulphonation of benzene is a reversible reaction.
-The mixture of sulphur trioxide and sulphuric acid is known as fuming sulphuric acid. We know that oxygen has high electronegativity. Thus, oxygen in sulphuric acid pulls an electron toward itself and generates an electrophile SO3. The electrophile SO3 then reacts with benzene to form benzene sulphonic acid.
The sulphonation of benzene occurs in three steps: 1. Formation of an electrophile, 2. Formation of a carbocation intermediate and 3. Removal of proton from the carbocation intermediate.
The sulfonation reaction of benzene is as follows:
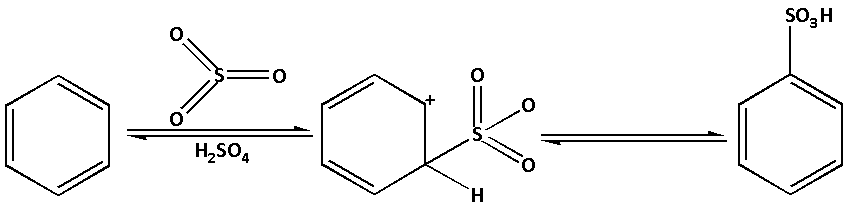
-Thus, the statement ‘SO3 is an electrophile in the sulphonation of benzene’ is true.
Thus, the correct option is (A).
Note: Remember that benzene is a planar molecule and has a cloud of delocalised electrons above the plane of the ring. Thus, benzene is rich in electrons. As a result, benzene is highly attractive towards the species that are electron deficient i.e. electrophiles. Thus, benzene undergoes electrophilic substitution reactions.
