Question
Question: \(\text{PC}{{\text{l}}_{5}}\) has a shape of trigonal bipyramidal whereas \(\text{I}{{\text{F}}_{5}}...
PCl5 has a shape of trigonal bipyramidal whereas IF5 has the shape of a square pyramid. It is due to
A. presence of an unshared electron pair on I which is oriented so as to minimise the repulsion while in P in PCl5 has no unshared pair.
B. octet of P is complete while that of I is incomplete.
C. P and I are of different groups.
D. F and Cl have different degrees of repulsion.
Solution
Phosphorus has five electrons in the outermost shell and it can make five bonds with other molecules whereas iodine has 7 electrons and has a tendency to accept a pair of electrons so it forms mainly a single bond.
Complete answer:
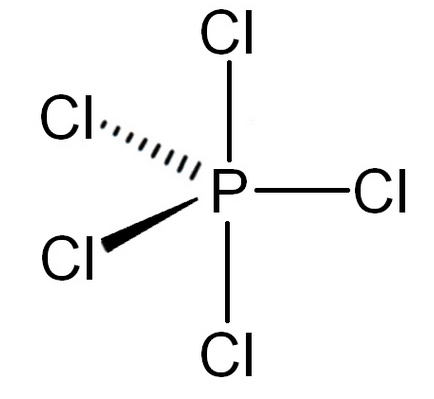
-In PCl5, the electronic configuration of phosphorus is 1s2 2s2 2p6 3s2 3p3 to form five bonds with chlorine electron form s-orbital is excited to the vacant d-orbital due to which phosphorus gains the ability to make 5 bonds.
-Now, it has a total of five unpaired electrons.
-So, the hybridisation of phosphorus is sp3d and the geometry will be trigonal bipyramidal.
-Because it has 5 bond pairs and 0 lone pairs.
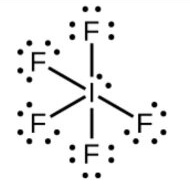
-In IF5, according to the electronic configuration of iodine two electrons from 5p-orbital are excited to move to the 5d-orbital.
-So, now iodine tends to make 5 bonds and along with it have one lone pair.
-The lone pair will cause the repulsion towards the bond pair and increases the bond angles that’s why it is oriented in such a way that it causes less repulsion.
-So, the hybridisation will be sp3d2and the geometry will be square pyramidal.
-The structure is:
So, the correct answer is “Option A”.
Note: The lone pair- lone pair repulsion causes maximum repulsion than lone pair-bond pair and lone pair-bond pair repulsion is more than the bond pair-bond pair repulsion.
