Question
Question: \({{\text{P}}_{\text{4}}}{{\text{O}}_{{\text{10}}}}\)on reacting with water does not form A. tetra...
P4O10on reacting with water does not form
A. tetra metaphosphoric acid.
B. phosphorous acid.
C. orthophosphoric acid.
D. pyrophosphoric acid.
Solution
The reaction ofP4O10with water is known as hydrolysis reaction. We will form hydrolysis products of P4O10 which will contain phosphorus, hydrogen and oxygen atoms. The oxidation state of the product will remain the same as in reactant.
Complete answer
The reactions with water which add water in the reactant are known as hydrolysis.
P4O10 reacts differently with cold and hot water. On reacting with cold water, P4O10gives metaphosphoric acid and on reacting with hot water, P4O10gives orthophosphoric acid.
P4O10 + 2H2O(cold)→4HPO3
HPO3is known as metaphosphoric acid. Four molecules join to form a tetramer.
The structure of metaphosphoric acid is as follows:
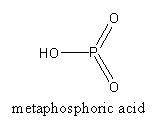
P4O10 + 6H2O(hot)→4H3PO4
HPO3is known as orthophosphoric acid.
The structure of orthophosphoric acid is as follows:
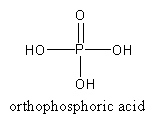
By the hydrolysis of P2O3, phosphorous acid forms.
P2O3 + 3H2O→2H3PO3
The structure of phosphorous acid is as follows:
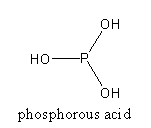
By the heating of two molecules of orthophosphoric acid, a molecule of pyrophosphoric acid forms.
2H3PO4→ΔH4P2O7
The structure of pyrophosphoric acid is as follows:
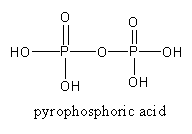
So, P4O10on reacting with water gives orthophosphoric acid and metaphosphoric acid.
So, P4O10on reacting with water does not form phosphorous acid and pyrophosphoric acid.
Therefore, option (B) phosphorous acid and (D) pyrophosphoric acid, are correct.
Note: Reactant and hydrolysis product both have phosphorus in the same oxidation state. Oxidation state of phosphorus in orthophosphoric acid and metaphosphoric acid is +5. The oxidation state of phosphorus inP4O10 is also +5. The oxidation state of phosphorus in phosphoric acid is +3. The oxidation state of phosphorus in pyrophosphoric acid is also +5 but is produced by heating of orthophosphoric acid not be hydrolysis of P4O10.
