Question
Question: \[{{\text{P}}_{4}}{{\text{S}}_{3}}\] has 6 P-S and ____ P-P bonds....
P4S3 has 6 P-S and ____ P-P bonds.
Solution
Phosphorus sesquisulfide, P4S3, an inorganic compound, with an orthorhombic crystal structure. By studying its geometry, we can determine the number of different types of bonds it has.
Complete Solution :
P4S3, a yellow solid, is one of the two commercially produced phosphorus sulphides. It is a major component of the strike-anywhere matches. Depending on purity of the compound obtained samples can appear yellow-green to grey. Phosphorus sesquisulphide, free from yellow and white phosphorus appears as a perfect yellow crystalline solid.
- This compound has a trigonal pyramidal geometry, having a triangular base made with phosphorus at each corner of the triangle. Each of the phosphorus on the triangular is perpendicularly attached to a sulphur atom. All these sulphur atoms are further attached to a single trivalent phosphorus atom as shown in the figure given below. It is a derivative of the tetrahedral phosphorus unit from insertion of sulphur into three P-P bonds. The P-S and P-P bond lengths are 2.090A∘ and 2.235A∘ respectively.
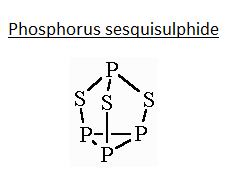
Thus, from the image and explanation given we infer that 6 P-S and 3 P−P bonds are present in the above compound.
Note: P4S3 compound is made by melting phosphorus and sulphur together at high temperatures and they form the mixed crystals of one dissolved in the other. Excess sulphur gives phosphorus penta-sulphide (P4S10). Its flash point is about 100∘C .
- It can be easily ignited by friction. It also forms sulphur dioxide and phosphorus penta-oxide during combustion with oxygen. It reacts with water to form phosphoric acid which is a corrosive material. It is also used to make matches and in the manufacture of other chemicals.
