Question
Question: \({\text{FeC}}{{\text{l}}_{\text{3}}}{\text{.6}}{{\text{H}}_{\text{2}}}{\text{O}}\) is actually: (...
FeCl3.6H2O is actually:
(A)- [Fe(H2O)6]Cl3
(B)- [Fe(H2O)5Cl]Cl.H2O
(C)- [Fe(H2O)4Cl2]Cl.2H2O
(D)- [Fe(H2O)3Cl3].3H2O
Solution
Given compound is an inorganic complex, which is chemically known as ferric trichloride hexahydrate. In the complex may or may not be all atoms will present inside the coordination sphere.
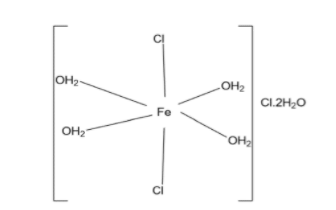
Complete answer: In the given complex iron (Fe) is present in the +3 oxidation state, it means iron will remove 3 electrons from its outermost shell to get stable electronic configuration. And FeCl3.6H2O is actually present in the [Fe(H2O)4Cl2]Cl.2H2O form.
-Two chlorine atoms are present inside the coordination sphere and one chlorine atom is present outside the coordination sphere in the ionization sphere.
-Four water molecules are present inside the coordination sphere and two water molecules are present outside the coordination sphere in the ionization sphere.
-Structure of complex is octahedral.
-Two chlorine atoms are present in 1800to each other in the octahedral structure and four water molecules are present in 900to each other.
Structure of given complex is shown as follow:
-Option (B) will not be the answer because there are not all 3 chlorine atoms present according to the given complex.
Hence, option (C) is the correct answer.
Additional information: Ferric trichloride hexahydrate is a yellow-orange color crystal which is hygroscopic in nature, it means they easily attract water molecules from surroundings in it.
Note: Here some of you may attach the two chlorine atoms anywhere in the coordination sphere rather than the given position, but that will be wrong because chlorine atom has high trans effect than water molecule that’s why the 2nd chlorine atom is always present at 1800 to the 1st chlorine atom.
