Question
Question: Tetrachloromethane is the IUPAC name of: (A) carbon tetrachloride (B) carbon hexachloride (C) ...
Tetrachloromethane is the IUPAC name of:
(A) carbon tetrachloride
(B) carbon hexachloride
(C) carbon pentachloride
(D) none of these
Solution
The IUPAC nomenclature of organic chemistry is a method of having organic compounds.
Its full form is the International union of pure and applied chemistry.
Step by step answer: We can follow the following steps while naming the compound.
The given compound is CCl4
This is halogen derivatives of alkane
The alkane is methane (CH4)and all H-atoms of methane is replaced by Ci-atoms.
Since IUPAC name of alkyl halide is halo alkane
There are four Cl-atoms in the compound.
∴its name is given as→tetrachloromethane
Molecule has one C-atom and four Cl-atoms
∴common name for compound is carbon tetrachloride.
The suffix tetra is used for Number four.
Therefore, from the above explanation the correct option is (A) carbon tetrachloride.
Additional Information: Tetra chloromethane is halogen derivatives of methane and obtained by chlorination of methane in presence of sunlight.
CH4+Cl2sunlightCH3Cl+HCl
Methane methyl chloride
CH3ClsunlightCH2Cl2+HCl
Methane dichloride
CH2Cl2+Cl2sunlightCHCl3+HCl
Chloroform
CHCl3sunlightCCl4+HCl
Carbon tetrachloride
H-atoms of methane replace one by one.
This process takes place by free radical mechanisms.
Since all four atoms are the same (Cl-atoms, present around the C-atom, therefore it forms regular tetrahedral geometry.
Cl-atoms are present at vertices of regular tetrahedron and angle between bond is 1090281
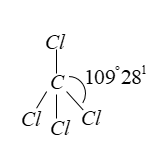
Note: IUPAC is a universally recognized authority on chemical nomenclature and terminology.
IUPAC develops uniform and consistent nomenclature and terminology for chemical substances.
