Question
Question: Tautomerism is exhibited by: A)\[{C_6}{H_5} - CH = CH - OH\] B) C6H5−CH=CH−OH
B)
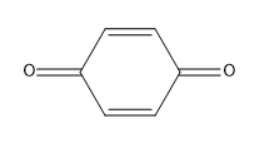
C)
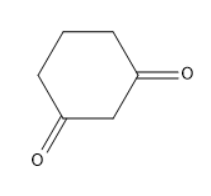
D)None of the above
Solution
The structures with the same molecular formula but difference in the hydrogen attachment can be called tautomerism. The two forms are keto form and enol form. Keto form can be also known as aci-form. in which the proton undergoes transfer to form keto and enol form.
Complete answer:
The chemical compounds with the same molecular formula but different structures are called isomers and the phenomenon is known as isomerism. Tautomerism is one of the types of structural isomerism. Ketone and enol are the two forms of tautomerism. Keto form can also be known as aci-form.
In the given option A, it is an enol form as both alkene and alcohol are there. The keto form is as follows:
C6H5−CH=CH−OH⇔C6H5−CH2−CHO
Thus, it exhibits tautomerism.
In the given option B, it is a carbonyl compound with conjugation of double bond.
It does not convert into an enol form.
Thus, it does not exhibit tautomerism.
In the given option C, it is a keto form.
The enol form for the given compound will be as follows:
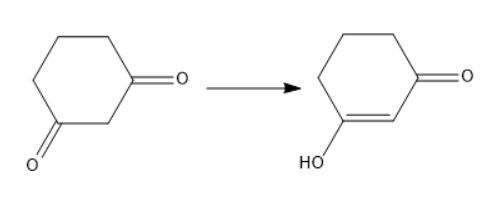
Thus, it exhibits tautomerism.
Tautomerism is exhibited by A and C.
Options A and C are the correct options.
Note:
The presence of alpha-hydrogen compounds will only show tautomerism. In the both A and C options there is an alpha hydrogen atom. But in option B there is no sp3 alpha hydrogen atom. Thus, it does not exhibit tautomerism. There is a presence of sp3 alpha hydrogen atom in both options A and C.
