Question
Question: A human adult breathes in approximately 0.50 $dm^3$ of air at 1.00 bar with each breath. If an air t...
A human adult breathes in approximately 0.50 dm3 of air at 1.00 bar with each breath. If an air tank holds 100 dm3 of air at 200 bar, how many breathes (in order of 104) the tank will supply?
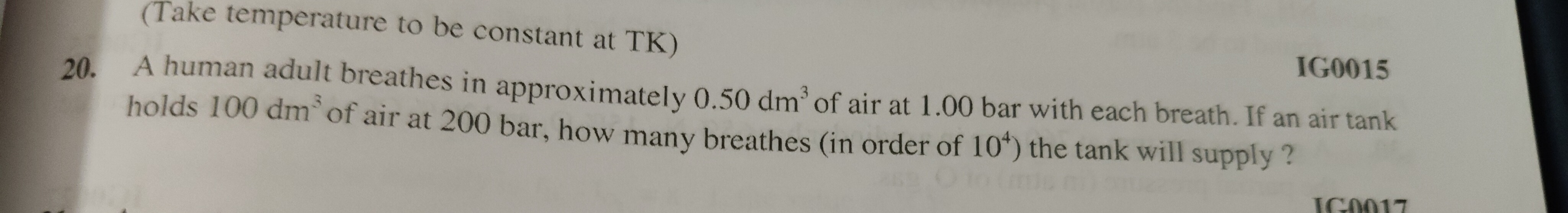
4.0
0.4
40.0
0.04
4.0
Solution
At constant temperature, the amount of gas is proportional to the product of pressure and volume (PV). The total amount of gas available from the tank is proportional to Ptank×Vtank. The amount of gas consumed per breath is proportional to Pbreath×Vbreath. The number of breaths (N) is the ratio of the total amount of gas to the amount per breath: N=Pbreath×VbreathPtank×Vtank
Given: Pbreath=1.00bar Vbreath=0.50dm3 Ptank=200bar Vtank=100dm3
N=(1.00bar)×(0.50dm3)(200bar)×(100dm3) N=0.5020000 N=40000
The question asks for the number of breaths in order of 104. 40000=4.0×104. Thus, the number of breaths is 4.0, when expressed in units of 104.
