Question
Question: Synergic back-bonding is absent in transition metal carbonyls. If true enter 1, else enter 0....
Synergic back-bonding is absent in transition metal carbonyls. If true enter 1, else enter 0.
Solution
“Synergic bonding is also known as π-back bonding”. It is usually used in the concept of organometallic chemistry where there is a transition metal centre and good π –electron acceptor ligands like CO.
Complete step by step answer:
In organometallic chemistry there is an involvement of two types of bonding interactions at play.
Firstly, ligands will donate the electrons to empty orbitals of the metal which shows a typical ligand-metal interaction. After that if the metal has filled d-orbitals, a second interaction will occur, where the electrons are donated to empty orbitals of ligands from the filled d-orbitals of the metal.
Together, these two interactions allow the formation of synergic bonding.
This synergic back-bonding makes the transition metal carbonyl complexes as more stable than the other coordination complexes. A suitable example for synergic back-bonding is tetracarbonyl nickel complex.
The color of the transition metal carbonyl complexes is going to change by the presence of synergic-back bonding.
All ligands won't form back bonding in transition metal complexes.
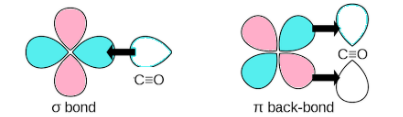
All ligands won't form back bonding in transition metal complexes. Synergic back-bonding is present in transition metal carbonyl complexes.
The given statement is true. So, the answer we have to write is “0”.
Note: Don’t be confused with the words back-bonding and synergic back-bonding, both are a bit different. Synergic back-bonding is only possible in case of transition organometallic complexes. Organometallic compounds are those organic compounds that have at least one metal atom attached to it.
