Question
Question: Symbol D stands for: A. Dextrorotatory, which rotates P.P.L towards right. B. Dextrorotatory, wh...
Symbol D stands for:
A. Dextrorotatory, which rotates P.P.L towards right.
B. Dextrorotatory, which rotates P.P.L toward left.
C. Relative configuration with respect to lactic acid taken as standard.
D. Relative configuration with respect to glyceraldehyde taken as standard and (OH) group is on the right side.
Solution
To solve this we must know that D is known as a dextrorotatory form. The monosaccharides are named as D (dextrorotatory) or L (laevorotatory) based on the stereochemical configuration.
Complete answer:
We know that the monosaccharides are named as D (dextrorotatory) or L (laevorotatory) based on the stereochemical configuration.
The monosaccharides are named based on the stereochemical configuration of glyceraldehyde.
The D (dextrorotatory) enantiomer of glyceraldehyde is a naturally occurring enantiomer. It is represented as (+)-glyceraldehyde or D- glyceraldehyde.
We know that a tetrahedral carbon atom is represented in Fischer projection by two crossed lines. The Fischer projection for D-glyceraldehyde is as follows:
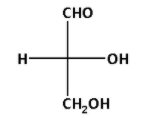
In the Fischer projection for D-glyceraldehyde, we can see that the most oxidised carbon atom lies at the top. The hydroxyl (OH) group is at the lowest chirality centre and points towards right.
All the sugars in which the hydroxyl (OH) group is at the lowest chirality centre and points towards right are known as D-sugars. In relative configurations, glyceraldehyde is taken as standard.
The D notation describes the configuration at only one chirality centre and it does not describe the other chirality centres present.
Thus, symbol D stands for relative configuration with respect to glyceraldehyde taken as standard and (OH) group is on the right side.
Thus, the correct option is (D) relative configuration with respect to glyceraldehyde taken as standard and (OH) group is on the right side.
Note:
The exact opposite of D-sugars are L-sugars. All the sugars in which the hydroxyl (OH) group is at the lowest chirality centre and points towards left are known as L-sugars. In both D- and L-sugars the most oxidised carbon atom i.e. the aldehyde group (CHO) lies at the top.
