Question
Question: Suppose your laboratory has only \(C{H_3}OH\) as organic compounds and all inorganic chemicals are a...
Suppose your laboratory has only CH3OH as organic compounds and all inorganic chemicals are available. How will you synthesize the following alcohol.(SEE FIGURE)
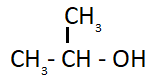
Solution
Hint: The given compound is isopropyl alcohol or 2− propanol. We can synthesize isopropyl alcohol from methanol in a laboratory where all inorganic chemicals are available. We can obtain it with the help of many reactions. We have to perform these reactions one after another in order to get 2− propanol. These reactions are as follows: 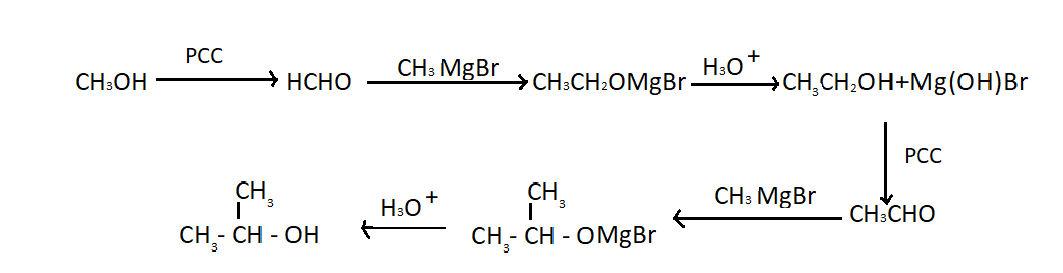
Complete answer:
Lets understand each reaction one by one in order to get the isopropyl alcohol. First of all methanol has reacted with PCC (pyridinium chlorochromate) which oxidises primary alcohol to aldehydes so here we get formaldehyde when PCC oxidizes methanol. Methyl magnesium bromide or CH3MgBr is a Grignard reagent when it reacts with formaldehyde it gives ethanol(CH3CH2OH) which is a 10 alcohol. Now we will repeat the above reaction with ethanol in order to get 20 alcohol which is isopropyl alcohol. One more point to mention here that Grignard reagents is not a pure organic compound as it is prepared by treating halides with magnesium metals. Ethers are used to stabilize the organo-magnesium compound. So we can use this compound in the preparation of isopropyl alcohol as it is not a pure organic compound.
Note: It is a very useful alcohol. isopropyl alcohol is colourless liquid with slight odour of rubbing alcohol. It has antibacterial properties and its hydroxyl groups can form weak bonds known as hydrogen bonds that are helpful in holding the molecules. It is also known as 2− propanol. It has a lower dielectric constant than ethanol and water that means it has ability to shield opposite charges and keep them separated.
