Question
Question: Sulphurous acid turns -------. A.Blue litmus into red B.Red litmus into blue C.No change to an...
Sulphurous acid turns -------.
A.Blue litmus into red
B.Red litmus into blue
C.No change to any of the litmus paper
D.Can’t say
Solution
Litmus paper can be prepared from a filter paper. The filter paper has to be treated with a natural soluble dye called lichens. The piece of filter paper that is obtained is called litmus.
There are two types of litmus papers, blue litmus paper and red litmus paper.
Complete answer:
The given chemical in the question is sulphurous acid.
The molecular formula of sulphurous acid isH2SO3.
The structure of the H2SO3 is as follows
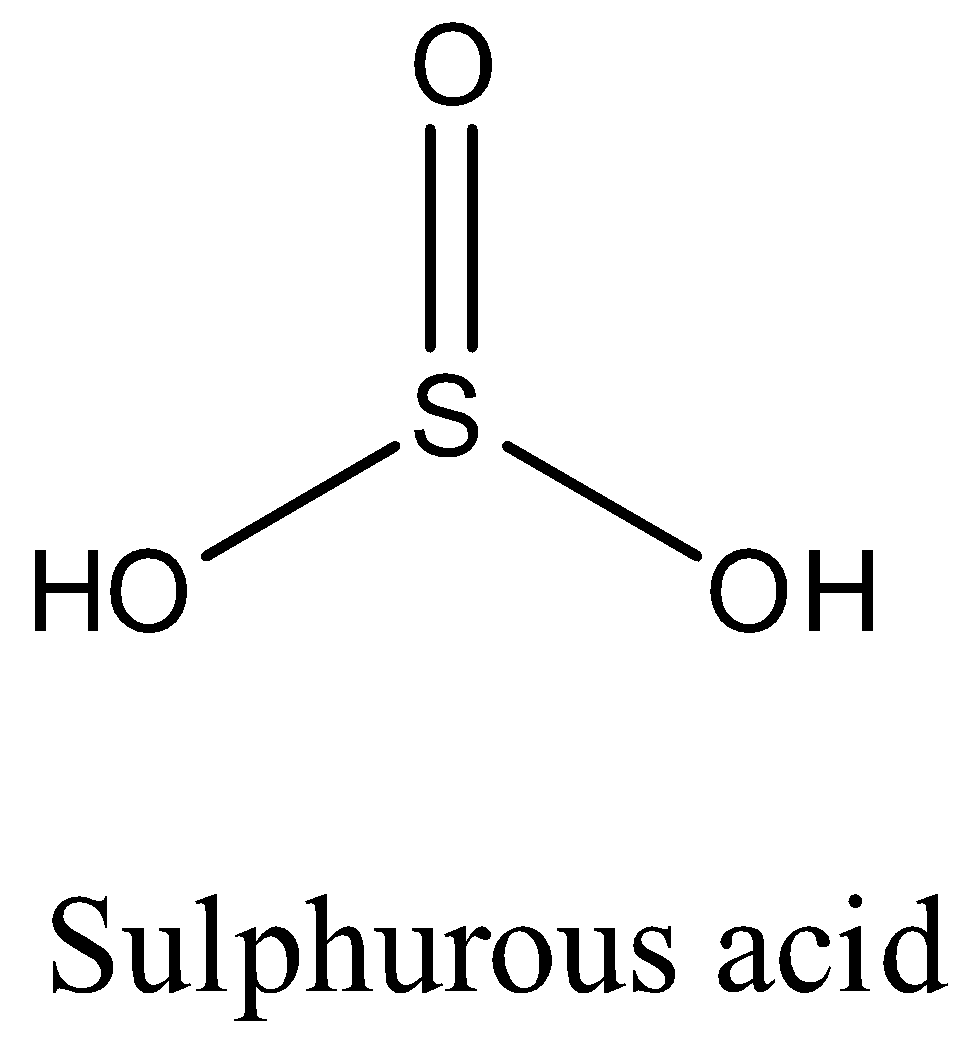
Sulphurous acid releases two hydrogens when dissolved in water then H2SO3 is an acid.
Acid turns blue litmus to red litmus and base turns red litmus to blue litmus.
Therefore H2SO3 turns blue litmus paper into red litmus paper.
So, the correct option is A.
Additional information:
Sulfurous acid is a weak acid and called dibasic acid also because it releases two hydrogens when dissolved in water.
Sulfurous acid and its respective salts are used as dominant reducing and disinfecting agents.
Sulfurous acid is used as a mild bleaching agent and can be used in place of chlorine to treat the materials that are sensitive towards chlorine.
Note:
Sulphurous acid can be prepared by treating sulphur dioxide with water.
SO2+H2O→H2SO3
Sodium sulfite is the most important salt of Sulphurous acid. Sodium sulfite can be easily prepared by treating sulphurous acid with base.
H2SO3+2NaOH→Na2SO3+2H2O
Molecular formula of Sodium sulfite is Na2SO3.
