Question
Question: Suggest reasons why the BF bond lengths in \(B{{F}_{3}}\)(130 pm) and \(B{{F}_{4}}^{-}\)(143 pm) dif...
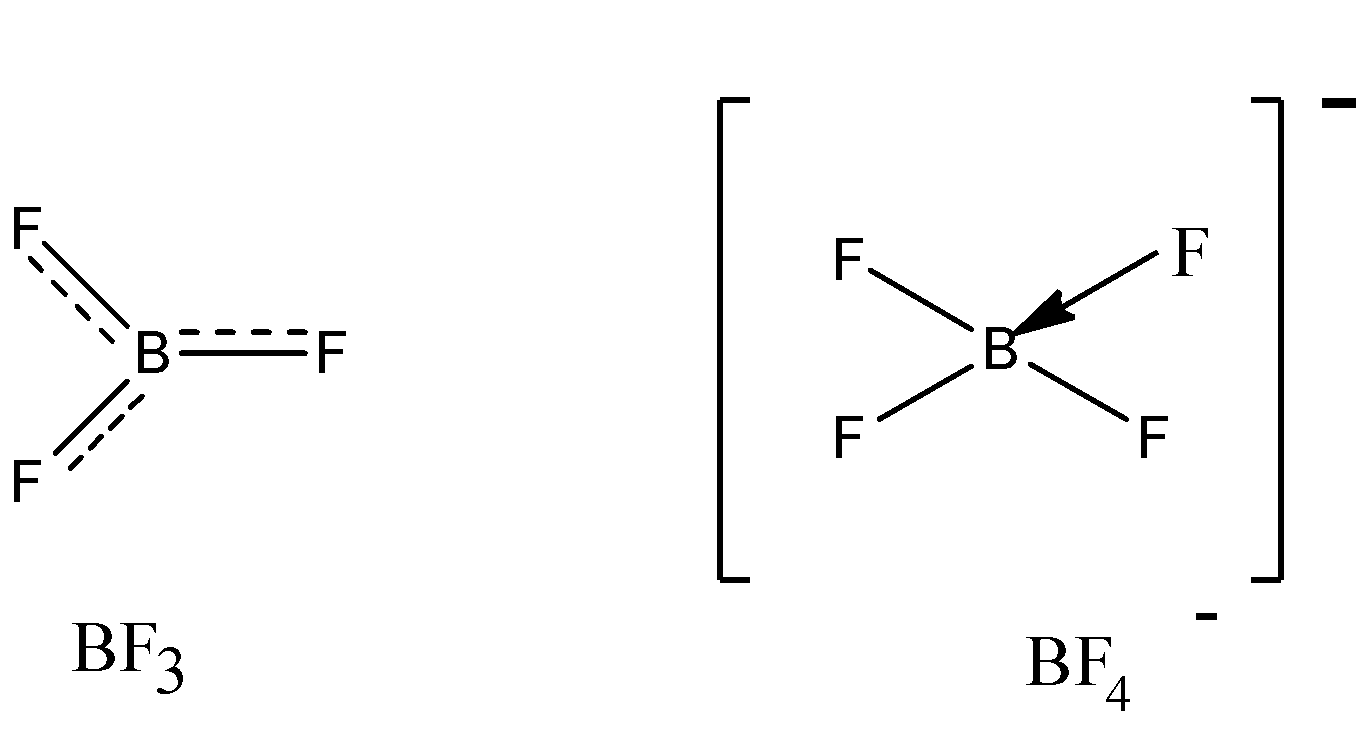
Suggest reasons why the BF bond lengths in BF3(130 pm) and BF4−(143 pm) differ.
Solution
BF3 molecule involves backbonding, which induces a minimal double bond character, and due to this, bond length is less.
Complete answer:
In order to answer the question, we need to learn about the nature of both the compounds and their hybridisation nature. Let us talk about sp2 and sp3hybridisation:
sp2Hybridisation: In sp2 hybridisation one s and two p (px and py) orbitals of one atom hybridize to give three equivalent sp2 hybrid orbitals. These three sp2 hybrid orbitals are directed towards the three corners of an equilateral triangle with an angle of 1200 and give a triangular geometry to the molecule. In BF3 boron is the central atom. Its electronic configuration in the ground state and excited state are one half filled 2s orbital and two half filled 2p orbital undergo hybridisation and produce three equivalent half filled sp2 hybrid orbitals. These hybrid orbitals are trigonal planar and are oriented at an angle of 1200 to each other. These three sp2 hybrid orbitals overlap with half filled 2p orbitals of three fluorine atoms to form three B-F sigma bonds. The resultant geometry of BF3 molecule is trigonal planar
sp3Hybridisation: In this hybridisation one s and three p-orbitals intermix to form sp3 hybrid orbitals of equivalent energy and identical shape. These four sp3 hybrid orbitals are directed towards the four comers of a tetrahedron separated by an angle of 109028′.
Now, let us come to our question.
In the BF3 molecule, there is back bonding present between B−F, which results in formation of partial double bond character. Electron deficiency is removed, and so bond length gets reduced.
In BF4−, the hybridization becomes sp3, the double bond character loose and single bond character takes place, which in turn increases the bond length. So, BF3 has a lesser bond length than BF4− due to backbonding.
Note:
It is to be noted that in the BF3 molecule, there is no resonance, however, delocalisation occurs. Resonance and delocalisation enhances stability of the compound.
