Question
Question: Sucrose on hydrolysis gives? (A) Glucose + Glucose (B) Glucose + Galactose (C) Glucose + Fruct...
Sucrose on hydrolysis gives?
(A) Glucose + Glucose
(B) Glucose + Galactose
(C) Glucose + Fructose
(D) Glucose + Lactose
Solution
Sucrose is a disaccharide made by the reaction between two aldohexose. Draw the expanded structure of sucrose. Now try to split the complex structure into two monosaccharides such that both compounds have 6 carbon atoms. Now identify the compounds obtained and then write the IUPAC name for the same.
Complete step by step answer:
Sucrose is considered as common sugar. Sucrose is produced naturally in plants from which the edible table sugar is refined. The molecular formula for sucrose is C12H22O11. We will now draw the expanded structure of a sucrose molecule.
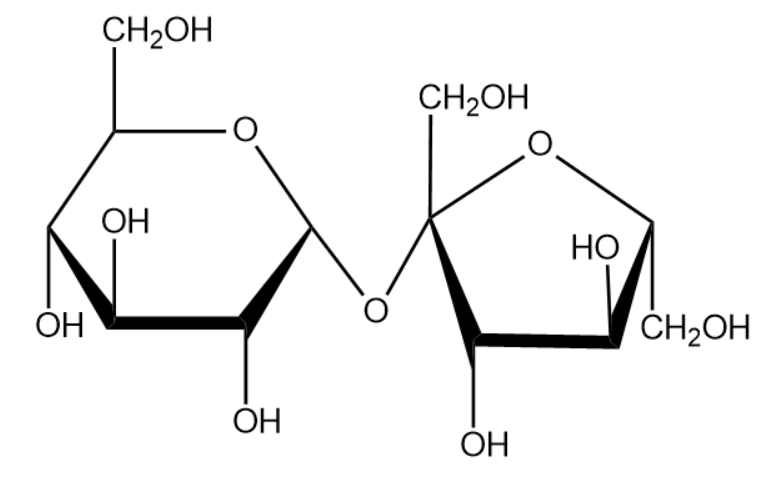
From the above structure we can identify the monosaccharide that help to from the compound sucrose. The structure compounds are given below:
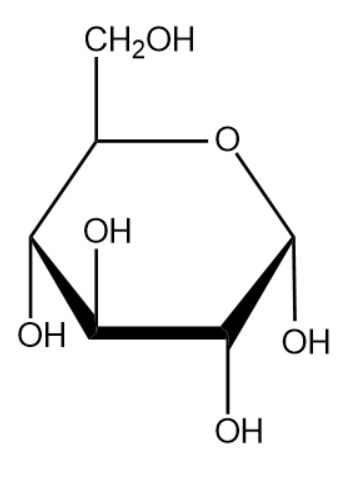
The name of the above monosaccharide is glucose.
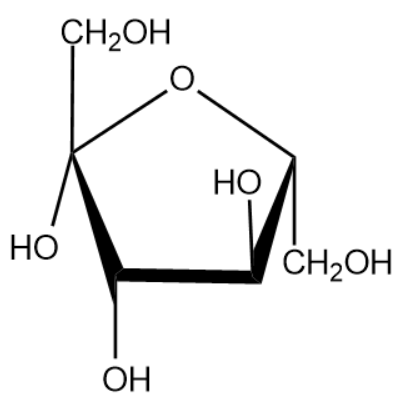
The name of the above monosaccharide is fructose.
From the above statements we can conclude that sucrose on hydrolysis will give the respective monosaccharides, glucose and fructose. So, the correct answer is “Option C”.
Note: It is important to know that sucrose is dextrorotatory in nature. However, upon hydrolysis the products obtained are dextrorotatory glucose and laevorotatory fructose. Since the rotation of fructose is much more than that to glucose, the mixture as a whole is considered laevorotatory. Since, the hydrolysis of sucrose brings a significant change in the type of rotation to the mixture, i.e. from dextrorotatory to laevorotatory, it is also called as invert sugar.
