Question
Question: Identify the pair in which the specified bond length of first is greater than second. $PCl_3F_2$, $P...
Identify the pair in which the specified bond length of first is greater than second. PCl3F2, PF3Cl2: BLP−Cleq SO2Cl2, SO2F2: BLS=O BF3, BCl3: BLB−X X = F/Cl NO3−, NO2: BLN−O O3, O2: BLO−O CO, CO2: BLC−O
- How many of the following species have no d-orbitals involved in their hybridisation? XeF2, SnF2, PbCl2, SF4, CCl4, BF3, SO2Cl2, XeO2F2, XeOF4, POCl3
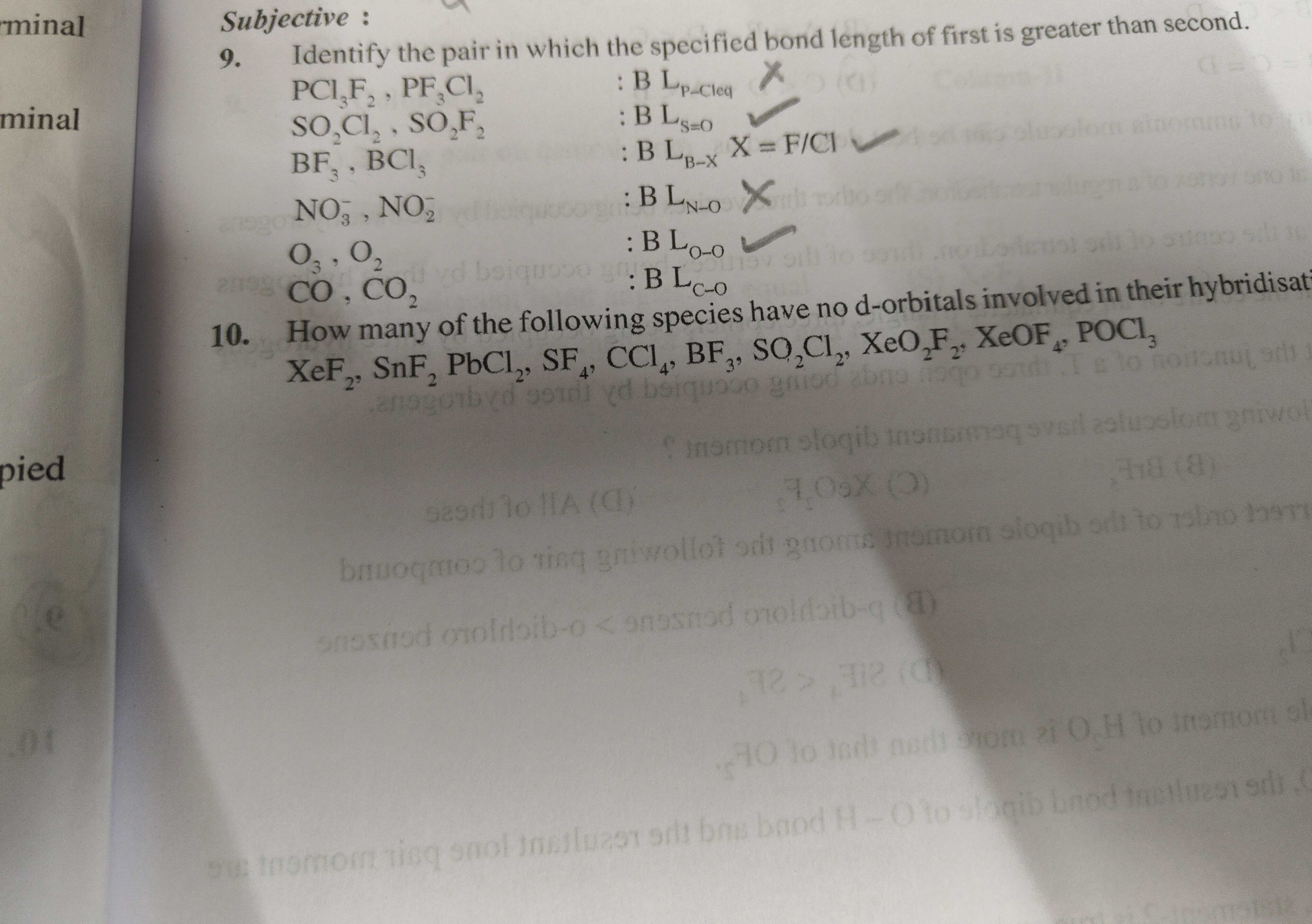
Question 9: The pairs in which the specified bond length of the first is greater than the second are:
- PCl3F2, PF3Cl2: BLP−Cleq
- SO2Cl2, SO2F2: BLS=O
- NO3−, NO2: BLN−O
- O3, O2: BLO−O
Question 10: 6
Solution
Solution for Question 9:
The question asks to identify the pairs in which the specified bond length of the first species is greater than that of the second species. We will analyze each pair:
-
PCl3F2, PF3Cl2: BLP−Cleq
- Both molecules adopt a trigonal bipyramidal (TBP) geometry. According to Bent's Rule, more electronegative substituents prefer positions with less s-character (axial positions in TBP), and less electronegative substituents prefer positions with more s-character (equatorial positions).
- In PCl3F2: The two more electronegative F atoms occupy axial positions. The three Cl atoms occupy equatorial positions. All three equatorial bonds are P-Cl. The s-character of the equatorial orbitals is equally distributed among these three P-Cl bonds.
- In PF3Cl2: The two F atoms occupy axial positions. The remaining F atom and the two Cl atoms occupy equatorial positions. So, the equatorial plane contains one P-F bond and two P-Cl bonds. Since F is more electronegative than Cl, the P-F bond will draw less s-character (more p-character) to itself compared to a P-Cl bond. Consequently, the two P-Cl equatorial bonds will have a larger share of the remaining s-character.
- Higher s-character in a bond leads to a shorter and stronger bond. Therefore, the P-Cl equatorial bonds in PF3Cl2 will be shorter than those in PCl3F2.
- Thus, BLP−Cleq in PCl3F2 > BLP−Cleq in PF3Cl2. This statement is TRUE.
-
SO2Cl2, SO2F2: BLS=O
- Both molecules have a tetrahedral geometry around the sulfur atom.
- Fluorine is significantly more electronegative than chlorine. The more electronegative F atoms in SO2F2 withdraw electron density from the central sulfur atom more effectively than the Cl atoms in SO2Cl2.
- This electron withdrawal makes the sulfur atom more electron-deficient. To compensate, sulfur strengthens its double bond character with oxygen (e.g., by increasing the p-character in the S-X bonds, leaving more s-character for the S=O bonds, or by enhancing back-donation from oxygen p-orbitals to sulfur d-orbitals, if d-orbitals are involved).
- Alternatively, according to Bent's Rule, the more electronegative F atoms will prefer hybrid orbitals with more p-character, leaving more s-character for the S=O bonds. More s-character leads to shorter bonds.
- Therefore, the S=O bond length in SO2F2 is shorter than in SO2Cl2.
- Thus, BLS=O in SO2Cl2 > BLS=O in SO2F2. This statement is TRUE.
-
BF3, BCl3: BLB−X (X = F/Cl)
- Both molecules are trigonal planar. We are comparing the B-F bond length in BF3 with the B-Cl bond length in BCl3.
- Fluorine is smaller and more electronegative than chlorine.
- In BF3, there is significant pπ-pπ backbonding from the lone pairs on F (2p orbital) to the empty p-orbital on B (2p orbital). This backbonding gives the B-F bond partial double bond character, leading to a shorter bond length.
- In BCl3, the pπ-pπ backbonding from Cl (3p orbital) to B (2p orbital) is much less effective due to the larger size of the Cl atom and poorer orbital overlap.
- Consequently, the B-F bond in BF3 is significantly shorter than the B-Cl bond in BCl3. (Experimental: B-F ~130 pm, B-Cl ~173 pm).
- Thus, BLB−X of BF3 < BLB−X of BCl3. The statement "first > second" is FALSE.
-
NO3−, NO2: BLN−O
- NO3− (Nitrate ion): It has three equivalent N-O bonds due to resonance. The resonance structures show one N=O double bond and two N-O single bonds. The average bond order is (2+1+1)/3 = 4/3 ≈ 1.33.
- NO2 (Nitrogen dioxide): It has two equivalent N-O bonds due to resonance. The resonance structures show one N=O double bond and one N-O single bond, with an odd electron. The average bond order is (2+1)/2 = 1.5.
- A higher bond order corresponds to a shorter bond length. Since the bond order of N-O in NO2 (1.5) is higher than in NO3− (1.33), the N-O bond in NO2 is shorter.
- Thus, BLN−O in NO3− > BLN−O in NO2. This statement is TRUE.
-
O3, O2: BLO−O
- O3 (Ozone): It has two equivalent O-O bonds due to resonance. The resonance structures show one O=O double bond and one O-O single bond. The average bond order is (2+1)/2 = 1.5.
- O2 (Oxygen molecule): It has a double bond (O=O). The bond order is 2.
- Since O2 has a higher bond order (2) than O3 (1.5), the O-O bond in O2 is shorter.
- Thus, BLO−O in O3 > BLO−O in O2. This statement is TRUE.
-
CO, CO2: BLC−O
- CO (Carbon Monoxide): It has a triple bond (C≡O). The bond order is 3.
- CO2 (Carbon Dioxide): It has two C=O double bonds. The bond order is 2.
- Since CO has a higher bond order (3) than CO2 (2), the C-O bond in CO is shorter.
- Thus, BLC−O in CO < BLC−O in CO2. The statement "first > second" is FALSE.
Pairs where the specified bond length of the first is greater than the second:
- PCl3F2, PF3Cl2: BLP−Cleq
- SO2Cl2, SO2F2: BLS=O
- NO3−, NO2: BLN−O
- O3, O2: BLO−O
Solution for Question 10:
The question asks to identify how many of the given species have no d-orbitals involved in their hybridization. This means we need to find species where the central atom uses only s and p orbitals for hybridization (i.e., sp, sp2, or sp3 hybridization). D-orbital involvement implies sp3d, sp3d2, sp3d3 hybridization.
To determine hybridization, we can use the steric number (SN = number of lone pairs + number of sigma bonds).
-
XeF2:
- Central atom: Xe. Valence electrons = 8.
- 2 F atoms form 2 sigma bonds.
- Remaining electrons = 8 - (2 * 1) = 6 electrons = 3 lone pairs.
- SN = 2 (sigma bonds) + 3 (lone pairs) = 5.
- Hybridization: sp3d. D-orbitals involved.
-
SnF2:
- Central atom: Sn. Group 14, Valence electrons = 4.
- 2 F atoms form 2 sigma bonds.
- Remaining electrons = 4 - (2 * 1) = 2 electrons = 1 lone pair.
- SN = 2 (sigma bonds) + 1 (lone pair) = 3.
- Hybridization: sp2. No d-orbitals involved.
-
PbCl2:
- Central atom: Pb. Group 14, Valence electrons = 4.
- 2 Cl atoms form 2 sigma bonds.
- Remaining electrons = 4 - (2 * 1) = 2 electrons = 1 lone pair.
- SN = 2 (sigma bonds) + 1 (lone pair) = 3.
- Hybridization: sp2. No d-orbitals involved.
-
SF4:
- Central atom: S. Valence electrons = 6.
- 4 F atoms form 4 sigma bonds.
- Remaining electrons = 6 - (4 * 1) = 2 electrons = 1 lone pair.
- SN = 4 (sigma bonds) + 1 (lone pair) = 5.
- Hybridization: sp3d. D-orbitals involved.
-
CCl4:
- Central atom: C. Valence electrons = 4.
- 4 Cl atoms form 4 sigma bonds.
- Remaining electrons = 4 - (4 * 1) = 0 electrons = 0 lone pairs.
- SN = 4 (sigma bonds) + 0 (lone pairs) = 4.
- Hybridization: sp3. No d-orbitals involved.
-
BF3:
- Central atom: B. Valence electrons = 3.
- 3 F atoms form 3 sigma bonds.
- Remaining electrons = 3 - (3 * 1) = 0 electrons = 0 lone pairs.
- SN = 3 (sigma bonds) + 0 (lone pairs) = 3.
- Hybridization: sp2. No d-orbitals involved.
-
SO2Cl2:
- Central atom: S. Valence electrons = 6.
- 2 O atoms form 2 double bonds (count as 2 sigma bonds for SN).
- 2 Cl atoms form 2 sigma bonds.
- Total sigma bonds = 2 (from O) + 2 (from Cl) = 4.
- Remaining electrons = 6 - (2 * 2) - (2 * 1) = 0 electrons = 0 lone pairs.
- SN = 4 (sigma bonds) + 0 (lone pairs) = 4.
- Hybridization: sp3. No d-orbitals involved.
-
XeO2F2:
- Central atom: Xe. Valence electrons = 8.
- 2 O atoms form 2 double bonds (count as 2 sigma bonds).
- 2 F atoms form 2 sigma bonds.
- Total sigma bonds = 2 (from O) + 2 (from F) = 4.
- Remaining electrons = 8 - (2 * 2) - (2 * 1) = 2 electrons = 1 lone pair.
- SN = 4 (sigma bonds) + 1 (lone pair) = 5.
- Hybridization: sp3d. D-orbitals involved.
-
XeOF4:
- Central atom: Xe. Valence electrons = 8.
- 1 O atom forms 1 double bond (count as 1 sigma bond).
- 4 F atoms form 4 sigma bonds.
- Total sigma bonds = 1 (from O) + 4 (from F) = 5.
- Remaining electrons = 8 - (1 * 2) - (4 * 1) = 2 electrons = 1 lone pair.
- SN = 5 (sigma bonds) + 1 (lone pair) = 6.
- Hybridization: sp3d2. D-orbitals involved.
-
POCl3:
- Central atom: P. Valence electrons = 5.
- 1 O atom forms 1 double bond (count as 1 sigma bond).
- 3 Cl atoms form 3 sigma bonds.
- Total sigma bonds = 1 (from O) + 3 (from Cl) = 4.
- Remaining electrons = 5 - (1 * 2) - (3 * 1) = 0 electrons = 0 lone pairs.
- SN = 4 (sigma bonds) + 0 (lone pairs) = 4.
- Hybridization: sp3. No d-orbitals involved.
Species with no d-orbitals involved in hybridization:
- SnF2 (sp2)
- PbCl2 (sp2)
- CCl4 (sp3)
- BF3 (sp2)
- SO2Cl2 (sp3)
- POCl3 (sp3)
There are 6 such species.
The final answer is 6
Explanation of the solution:
Question 9: To compare bond lengths, consider factors like bond order, atomic size, electronegativity, hybridization, and resonance.
- PCl3F2 vs PF3Cl2 (BLP−Cleq): In TBP geometry, equatorial bonds have more s-character. More electronegative atoms prefer less s-character. In PF3Cl2, the equatorial P-F bond (F is more electronegative than Cl) takes less s-character, leaving more s-character for the equatorial P-Cl bonds, making them shorter than P-Cl bonds in PCl3F2 where all equatorial positions are occupied by Cl. Thus, BLP−Cleq of PCl3F2 > BLP−Cleq of PF3Cl2.
- SO2Cl2 vs SO2F2 (BLS=O): More electronegative substituents (F) on the central atom (S) withdraw electron density, making the central atom more electron-deficient. This strengthens the pi-bonding with oxygen, leading to shorter S=O bonds in SO2F2 compared to SO2Cl2. Thus, BLS=O of SO2Cl2 > BLS=O of SO2F2.
- BF3 vs BCl3 (BLB−X): Significant pπ-pπ backbonding occurs in BF3 (from F 2p to B 2p), giving partial double bond character and shortening the B-F bond. This backbonding is much weaker in BCl3 (Cl 3p to B 2p) due to poor orbital overlap. Thus, BLB−F in BF3 < BLB−Cl in BCl3.
- NO3− vs NO2 (BLN−O): Bond length is inversely proportional to bond order. NO2 has an average N-O bond order of 1.5 (due to resonance), while NO3− has an average N-O bond order of 1.33. Higher bond order means shorter bond. Thus, BLN−O in NO3− > BLN−O in NO2.
- O3 vs O2 (BLO−O): O2 has a double bond (bond order 2). O3 has an average O-O bond order of 1.5 (due to resonance). Thus, BLO−O in O3 > BLO−O in O2.
- CO vs CO2 (BLC−O): CO has a triple bond (bond order 3). CO2 has double bonds (bond order 2). Thus, BLC−O in CO < BLC−O in CO2.
Question 10: Determine hybridization using steric number (SN = number of sigma bonds + number of lone pairs). If SN is 2, 3, or 4, hybridization is sp, sp2, or sp3, respectively, with no d-orbital involvement. If SN is 5, 6, or 7, hybridization is sp3d, sp3d2, or sp3d3, respectively, involving d-orbitals.
- XeF2: SN=5 (sp3d).
- SnF2: SN=3 (sp2).
- PbCl2: SN=3 (sp2).
- SF4: SN=5 (sp3d).
- CCl4: SN=4 (sp3).
- BF3: SN=3 (sp2).
- SO2Cl2: SN=4 (sp3).
- XeO2F2: SN=5 (sp3d).
- XeOF4: SN=6 (sp3d2).
- POCl3: SN=4 (sp3). Species with no d-orbital involvement are SnF2, PbCl2, CCl4, BF3, SO2Cl2, POCl3. Total 6 species.
