Question
Question: Identify the pair in which the specified bond length of first is greater than second. $PCl_3F_2, PF...
Identify the pair in which the specified bond length of first is greater than second.
PCl3F2,PF3Cl2:BLP−CleqX
SO2Cl2,SO2F2:BLS=O✓
BF3,BCl3:BLB−XX=F/Cl✓
NO3−,NO2:BLN−OX
O3,O2:BLO−O✓
CO,CO2:BLC−O
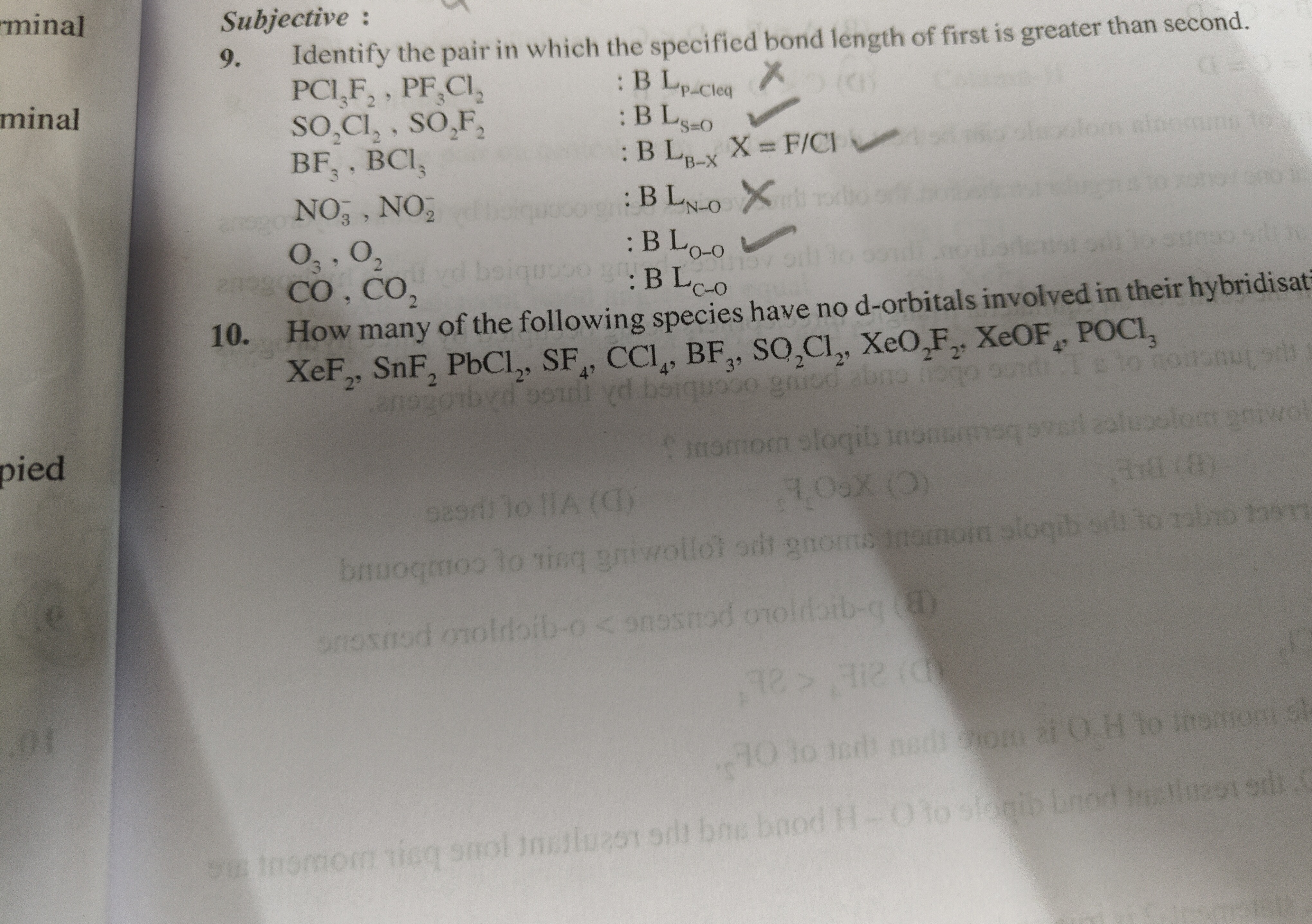
The pairs where the specified bond length of the first is greater than the second are:
- SO2Cl2,SO2F2:BLS=O
- NO3−,NO2:BLN−O
- O3,O2:BLO−O
Solution
Question 9 Explanation:
-
PCl3F2 vs PF3Cl2 (BLP−Cleq): In PCl3F2, F atoms are axial, Cl atoms are equatorial. In PF3Cl2, Cl atoms are equatorial, 1 F atom is equatorial, 2 F atoms are axial. The P-Cl equatorial bond in PF3Cl2 is slightly longer than in PCl3F2 due to the presence of a highly electronegative F atom in the equatorial plane competing for electron density. So, BLP−Cleq (PCl3F2) < BLP−Cleq (PF3Cl2).
-
SO2Cl2 vs SO2F2 (BLS=O): Fluorine is more electronegative than chlorine. In SO2F2, F atoms pull more electron density from S, making S more positive, thus strengthening and shortening the S=O bonds compared to SO2Cl2. So, BLS=O (SO2Cl2) > BLS=O (SO2F2).
-
BF3 vs BCl3 (BLB−X): Comparing B-F and B-Cl bond lengths. Chlorine is larger than fluorine, so B-Cl bond is inherently longer than B-F bond. Back-bonding in BF3 makes B-F even shorter. So, BLB−F (BF3) < BLB−Cl (BCl3).
-
NO3− vs NO2 (BLN−O): Bond length is inversely proportional to bond order. Bond order in NO3− is 1.33. Bond order in NO2 is 1.5. Higher bond order means shorter bond. So, BLN−O (NO3−) > BLN−O (NO2).
-
O3 vs O2 (BLO−O): Bond order in O3 is 1.5. Bond order in O2 is 2. So, BLO−O (O3) > BLO−O (O2).
-
CO vs CO2 (BLC−O): Bond order in CO is 3. Bond order in CO2 is 2. So, BLC−O (CO) < BLC−O (CO2).
