Question
Question: Study the experimental step up in figure and then answer the questions that follow: a) What phenom...
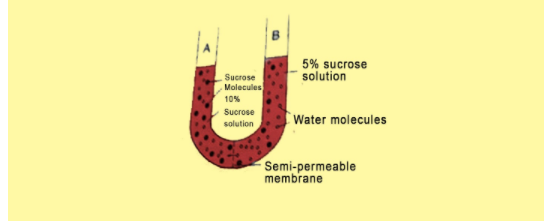
Study the experimental step up in figure and then answer the questions that follow:
a) What phenomenon is being studied by this setup?
b) Explain the phenomenon mentioned in (a) above
c)What is meant by ‘semipermeable’ membrane?
d)What will you observe in the setup after about half an hour? Give a reason for your answer.
Solution
The osmotic pressure is the pressure which is applied by the solution to prevent the inward motion of the pure solvent from the semi-permeable membrane or we can also say that it is a tendency of the liquid to take in a pure solvent across the semipermeable membrane by the process of osmosis.
Complete answer:
-In this setup osmotic potential can be studied.
-The osmotic potential is also called solute potential in plants. It is mostly negative in the plants and is zero when checked in distilled water.
-The semi-permeable membrane is the membrane that is present between the two different solutes in the setup. This membrane allows only particular ions and solutes to pass through it by the process of osmosis or facilitated diffusion.
- The potential energy in the water gets reduced due to the solutes which reduce the water potential. Solutes are able to dissolve in the water due to the binding of the hydrogen bonds. The amount of the potential energy of the water gets reduced when solutes are added to them. This results in a decrease in the osmotic potential as there is an increase in the solute concentration. So, the movement of the solutes will occur from the right side to the left side as the concentration of the solutes is more on the left side. After keeping the setup for an hour, we can observe an increase in the amount of solution on the left side and a decrease in the solution on the right side.
Note: The osmotic potential is the amount of decrease in the potential of the water when the solutes are added to it. This decrease in potential is directly proportional to the amount of solute added to the water. The osmotic potential acts opposite to the turgor pressure.
