Question
Question: Structures of \( N{(C{H_3})_3} \) and \( N{(Si{H_3})_3} \) are different. It is due to the fact that...
Structures of N(CH3)3 and N(SiH3)3 are different. It is due to the fact that:
(A) Silicon also uses d− orbitals in multiple bonding.
(B) In case of N(SiH3)3 , a lone pair N− atom is transferred to the empty d− orbitals of silicon (pπ−dπ overlapping)
(C) Both (A) and (B)
(D) None of the above
Solution
Si has vacant d− orbitals while C has no low-lying d− orbitals. The lone pair of nitrogen atoms resides on the p− orbital. There is a pπ−dπ back bonding between nitrogen and silicon atoms.
Complete answer:
Silicon has vacant d− orbitals and due to this in N(SiH3)3 , lone pair of N− atom is transferred to the empty d− orbitals of silicon (pπ−dπ overlapping) with electrons. Hence, N(SiH3)3 bond gains partial double bond character and its hybridisation becomes sp2 hybridized. So, it has a trigonal-planar structure. However, there is no such bond in N(CH3)3 because C has no d− orbital. It undergoes sp3 hybridization and its shape becomes a pyramidal structure.
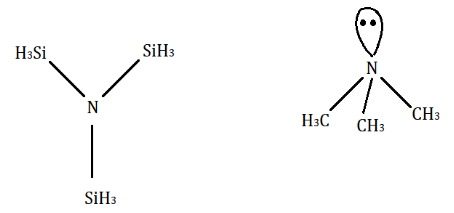
Hence, option C is correct.
Additional Information:
In N(SiH3)3 , the electronic configuration of Si is:
1s2,2s22p6,3s13px13py13pz1
Hence, Si has vacant d− orbitals. The filled 2p6 orbital of nitrogen overlaps with the vacant d− orbitals of Si to form pπ−dπ bond. This pπ−dπ bonding is fairly stable.
In Trimethylamine (N(CH3)3) , the lone pair is concentrated on the Nitrogen atom. But in case of Trisilyl amine (N(SiH3)3) , the lone pair on nitrogen gets delocalized onto the three Si atoms bonded to it. Hence, Trimethylamine is a stronger base as compared to Trisilyl amine as it can easily donate its lone pair to other atoms.
Trimethylamine is used in the synthesis of various plant growth regulators, herbicides, dye levelling agents and a number of basic dyes.
Note:
To understand back-bonding just imagine it as a sort of resonance between a lone pair and the other atom with a vacant d− orbital or p− orbital. When back bonding is stabilised in N(SiH3)3 , its bond angle increases. Thus, the bond length decreases and bond order increases. The bond angle of N(SiH3)3 is 120∘ while that of (N(CH3)3) is 107∘ .
