Question
Question: Structure of \(Xe{F_6}\) is octahedron with \(s{p^3}{d^3}\) hybridization of \(Xe\) . A.True B.F...
Structure of XeF6 is octahedron with sp3d3 hybridization of Xe .
A.True
B.False
Solution
We can find out the hybridization of an atom in a compound, utilizing a steric number. We can compute the steric number by summarizing the number of atoms attached to the center molecule and the solitary sets of electrons on the central metal particles. Tell us that on the off chance that the steric number is 4, at that point we state the molecule is in sp3 hybridization, on the off chance that the steric number is 3, at that point, it is sp2 hybridization, if the steric number is 2, at that point it is sp hybridization.
Complete answer:
We can draw the structure of XeF6 as,
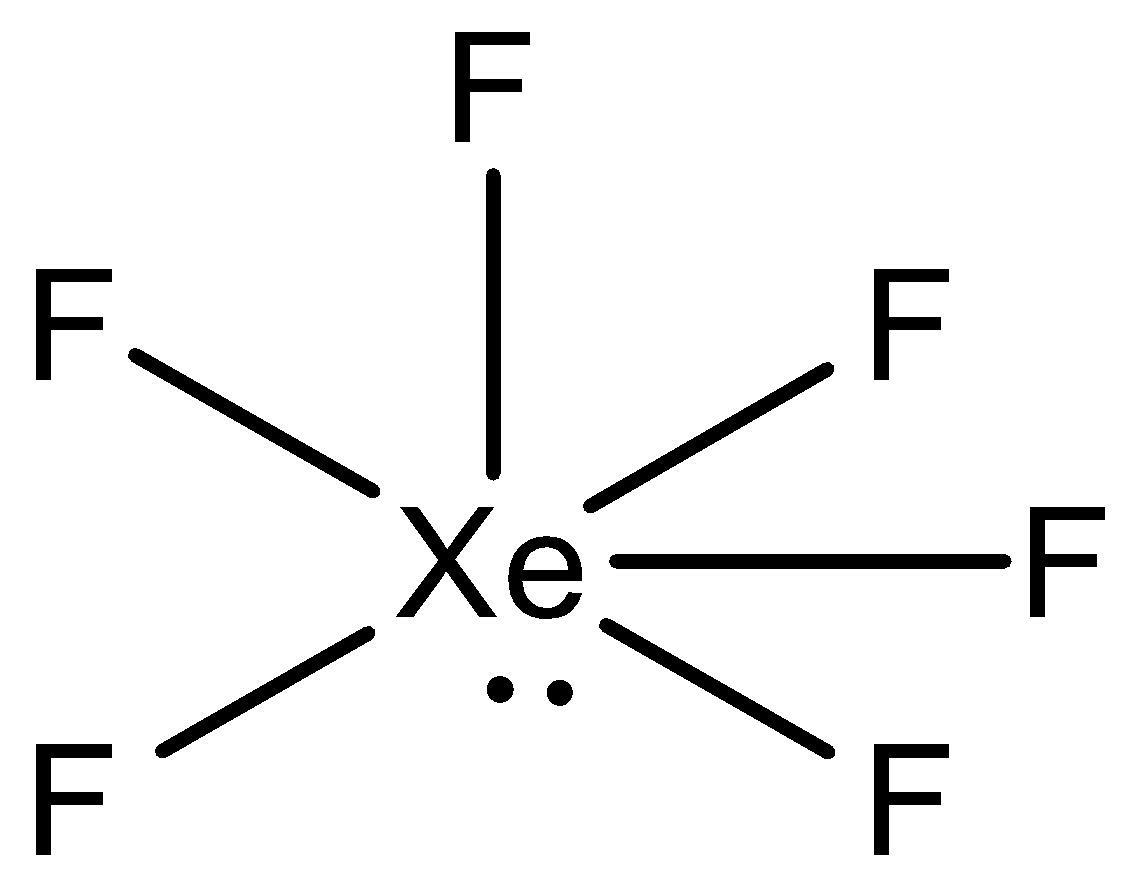
During the arrangement of XeF6 , xenon has eight electrons in its valence shell and it creates six bonds with the fluorine atoms. Further, the atoms will have one lone pair of electrons and six bond pairs. Presently on the off chance that we take the steric number, at that point, it will be seven. This can be deciphered as sp3d3 hybridization.
After hybridization, XeF6 molecular geometry would be distorted octahedral or square bipyramidal. What takes place here is that the atoms of fluorine atoms are located in the apexes of the octahedron while the lone pairs travel in the space to evade or decrease the repulsion.
Therefore, the option (B) is correct.
Note:
We have to remember that, XeF6 contains seven pairs of electrons. In that the number of bond pairs is six and the number of lone pairs is one. The number of electrons in the outermost shell of xenon is eight. When the fluorides of xenon have made electrons in the outermost shell of xenon get unpaired and are moved to empty 5d orbital.
