Question
Question: Structure of C in the given reaction sequence is: 
A.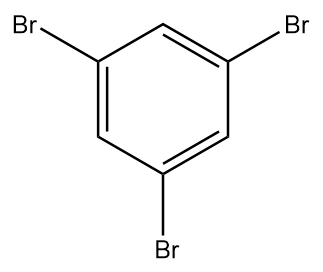
B.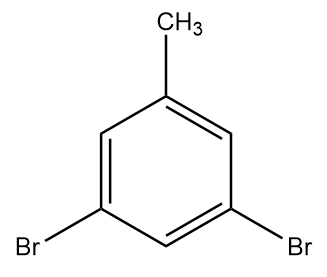
C.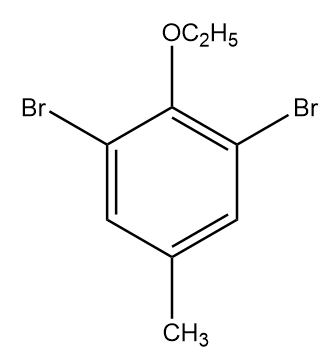
D.
Solution
Hint : Just like an alkene, the benzene ring which is an aromatic compound consists of a cloud of pi electrons and is composed of a single bond framework. Although the benzene ring forms a stable aromatic system due to resonance , the atoms are still available for reactions with strong electrophiles to undergo electrophilic substitution reaction.
Complete Step By Step Answer:
The mechanism for given reaction sequence is as follows:
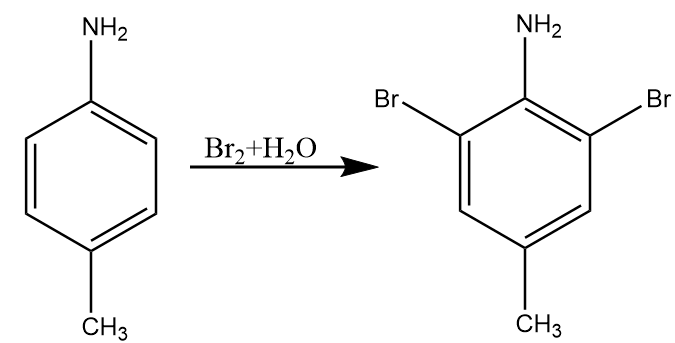
Step-1: The given reactant i.e., para-toluidine reacts with bromine water to undergo bromination. As aniline group is ortho para directing but para position is blocked by methyl group. So, bromine atoms will attack at both ortho positions of the ring and formation of product A i.e., 2,6-dibromo-4-methylaniline takes place. The reaction proceeds as follows:
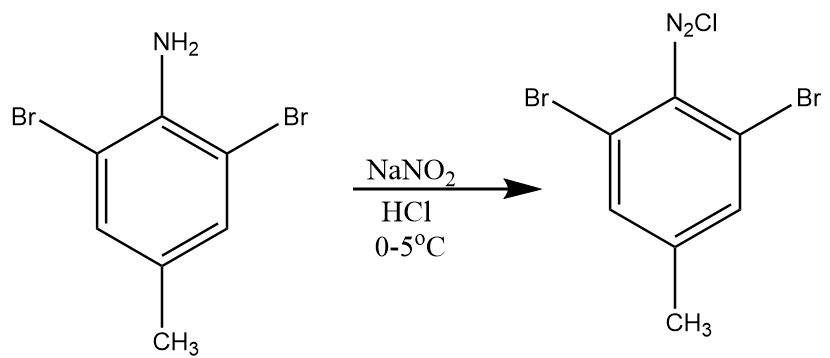
Step-2: In the presence of NaNO2 and HCl, the product A undergoes diazotization reaction and NH2 group will be converted into N2Cl under given conditions. So, the product B formed is 1-chloro-2-(2,6-dibromo-4methylphenyl) diazene. The reaction proceeds as follows:
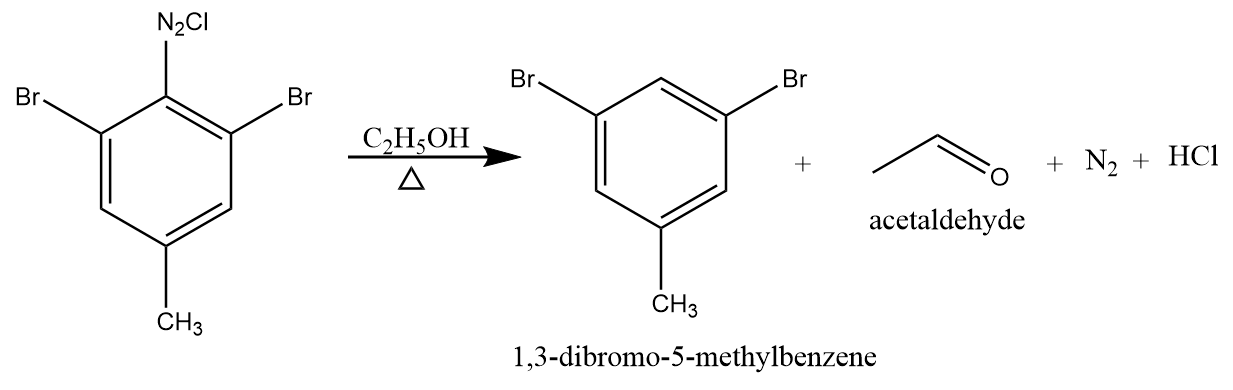
Step-3: On reaction of ethanol with product B, reduction of compound takes place and 1,3-dibromo-methyl benzene is formed as the final product along with the removal of acetaldehyde, nitrogen and hydrogen chloride. The reaction proceeds as follows:
Hence, the final product formed i.e., product C is 1,3-dibromo-5methylbenzene and it is structurally represented as:
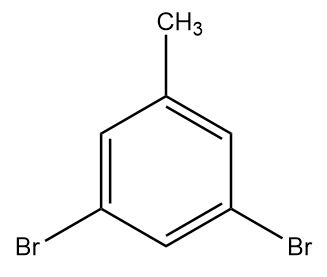
Therefore, option (B) is the correct answer.
Note :
It is important to note that in the reaction sequence, bromination and diazotization are type aromatic electrophilic substitution reaction i.e., an external electrophile replaced an electrophile from the benzene ring and formation of substituted aromatic product takes place whereas in last step, deamination of azo compound occurs to form final product.
