Question
Question: Strong reducing behaviour of \({H_3}P{O_2}\) is due to: a.) High oxidation state of phosphorus b...
Strong reducing behaviour of H3PO2 is due to:
a.) High oxidation state of phosphorus
b.) Presence of two −OH groups and one P−H bond
c.) Presence of one −OH group and two P−H bonds
d.) High electron gain enthalpy of phosphorus
Solution
In order to deal with this question first we will define the term reducing agent further according to its property and its structure we will find the required reason behind the strong reducing nature of hypophosphorous acid.
Complete step by step answer:
Reducing agent (also known as reductant or reducer) is an entity or compound that loses (or "donates") an electron in a redox chemical reaction to an electron receiver (oxidizing agent).
And a reduction agent is oxidized as electrons are lost in the redox reaction. Reducers have extra electrons (that is, they are reduced by themselves) in their pre-reaction states, whereas oxidizers lose electrons (that is, they are oxidized by themselves). A reducer usually occurs in one of the lowest potential oxidation states and is known as the donor of electrons. Earth elements, formic acid, oxalic acid, and sulphite derivatives are examples of reducing agents.
So, all oxyacids of phosphorus which have P−H bonds act as strong reducing agents. H3PO2 has two bonds hence, it acts as a strong reducing agent.
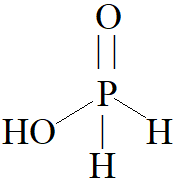
From the diagram it is quite clear that there is a presence of one −OH bond and two P−H bonds.
Hence, strong reducing behaviour of H3PO2 is due to the presence of one −OH group and two P−H bonds.
So, the correct answer is “Option C”.
Note: On comparing the reducing nature of the acids H3PO2, H3PO3 and H3PO4. We find that the oxidation number of P in H3PO2 and H3PO3 is +1 and +3 respectively, which can be further oxidised to the higher oxidation state while the P of the H3PO4 is having +5 oxidation state which is the highest oxidation state of the P. Hence both H3PO2 and H3PO3 can be act as reducing agent while H3PO4 can’t be used as reducing agent. Among H3PO2 and H3PO3 the H3PO2 is more reducing in nature because of the lowest oxidation number of the P.
H3PO2 (Hypophosphorous acid) is a compound having low-melting, colourless compounds and is soluble in water, dioxane, and alcohol. Due to its strong reducing agent it is commonly used to eliminate Cu, Hg and Ag etc. to test impurities like Nb, As and Ta, etc. Hypophosphorous acid also used in the manufacturing of some plastics (e.g. alkyl resins, polyamides, glycerol, polyester fibre, polyacrylonitrile, nylon fibres, epoxies, and fatty acid esters) as a decolourizing agent for colour stabilisation.
