Question
Question: Strong oxidizing agent used in purification of water. A. \(C{{l}_{2}}O\) B. \(N{{O}_{3}}^{-}\) ...
Strong oxidizing agent used in purification of water.
A. Cl2O
B. NO3−
C. NO2−
D. OF2
Solution
Oxidizing agent is strong if it can reduce itself to a high amount. Only then can the other atom present in the electrolyte be able to oxidize itself. For that to happen, the oxidizing agent is chosen such that the anion formed after its reduction is stable so as to not react with the compounds again.
Complete step by step solution:
-Based on the property of the compounds being able to dissociate into their ions, they are classified in 2 groups- electrolytes and non-electrolytes. Electrolytes are further classified as strong and weak electrolytes.
-Electrolyte is a compound which dissociates into its constituent ions, cation and anion in presence of DC current under the process of electrolysis.
-Many types of compound can be used as electrolytes but the most preferred compounds are acids, bases and salts. The electrolytes give redox reactions for dissociation into their ions.
-Electrolysis is a technique used to separate a compound in its different constituents with the help of DC current. It is used in non-spontaneous chemical reactions.
-The amount of voltage to be given depends on the type of the electrolyte used. The voltage is called decomposition potential.
-There are two electrodes, cathode and anode dipped in the electrolyte. They are connected to the battery. In acidified water, water is mixed with dilute sulfuric acid. This mixture acts as electrolyte.
-Cathode is the electrode where the cations are attracted. Reduction takes place at cathode. Anode is the electrode where the anions are attracted. Oxidation takes place at anode.
-There are two types of agents. One is the oxidizing agents and the other is the reducing agents. Oxidizing agents are those which oxidize the other atom by reducing themselves. Reducing agents are those that reduce the other atom by oxidizing themselves.
-Oxidizing agent is strong if it is present in its maximum oxidation state and can be reduced to form a stable ion such that the other species gets oxidized. Water also needs a strong oxidizing agent so that the oxygen atom oxidizes from -2 state to 0 state.
-In the given options, the oxidation state of the following central atoms are +1,+5,+3 and +2 respectively. So except for chlorine, all other atoms cannot be reduced easily so as to oxidize water.
-Chlorine has vacant d-orbitals which is absent in O and N and so it can show variable covalencies as
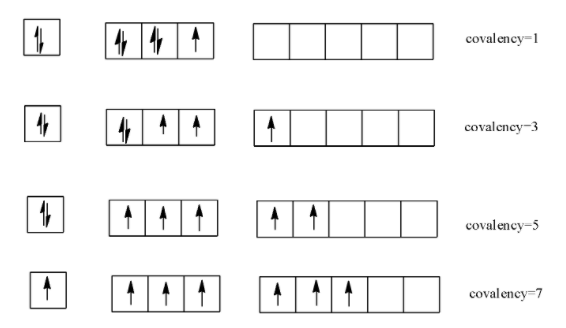
-Thus we see that chlorine can have different oxidation states and this makes it capable to form different compounds with different products. It reduces itself easily and oxidizes water and so it acts as a strong oxidizing agent.
Therefore the correct option is A.
Note: Ozone and hydrogen peroxide are also used for the purification of water as they can also reduce themselves easily from -1 to -2. Cl2O is reduced from +1 to -1 during the process and so is a good oxidizing agent and purifies the water by forming compounds with the impurity molecules.
