Question
Question: Strength of oleum is expressed in percentage. What is the percentage of free $SO_3$ in a sample of o...
Strength of oleum is expressed in percentage. What is the percentage of free SO3 in a sample of oleum labelled as 101.125%?
5%
Solution
For oleum, the percentage strength is defined as the weight of H2SO4 produced per 100 g of oleum after dilution with water. In oleum, the composition is taken as H2SO4⋅SO3 so that 100 g oleum yields 100 g H2SO4 before any free SO3 reacts with water. When water is added, the free SO3 reacts:
SO3+H2O→H2SO4
A sample labelled as 101.125% means 100 g oleum produces 101.125 g H2SO4 upon dilution, indicating an extra 1.125 g of H2O has combined with the free SO3.
Using the stoichiometry:
- 18 g of H2O reacts with 80 g of SO3 to form H2SO4.
Thus, the mass of free SO3 in 100 g oleum is:
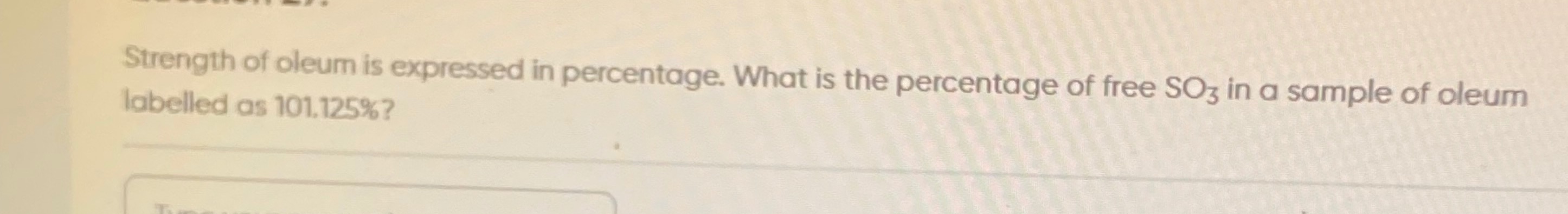
Free SO3=(1880)×1.125=1880×1.125=1890=5 g
So, the percentage of free SO3 is:
5%
Core Explanation:
Subtract the inherent acid (100 g) from the total acid after dilution (101.125 g) to get 1.125 g water. Then, using the ratio from the reaction (SO3+H2O→H2SO4) (80 g SO3 per 18 g H2O), compute free SO3 as (80/18)×1.125=5 g, i.e. 5%.
