Question
Question: Statement – I: When \(\text{C}{{\text{H}}_{\text{3}}}\text{I}\) and \({{\text{C}}_{\text{2}}}{{\text...
Statement – I: When CH3I and C2H5I react in the presence of Na/ dry ether, it forms three types of alkenes.
Statement – II: In Wurtz reaction Na acts as a reducing agent.
A) Statement I & II are correct. Statement – II is the correct explanation of Statement I.
B) Statement – I & II are correct. Statement – II is not a correct explanation of Statement I.
C) Statement – I is true but Statement – II is false.
D) Statement – I is false but Statement – II is true.
Solution
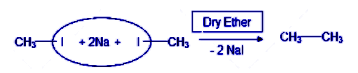
The reaction mentioned in the question is the Wurtz reaction. Wurtz reaction is the reaction of alkyl halides in the presence of dry ether and sodium metal to form alkanes.
Complete step by step solution:
The reaction involves a free radical formation as an intermediate. Firstly there is a single electron transfer from sodium metal to the alkyl halide. Then the alkyl halide forms a radical along with the sodium halide salt. Another sodium atom transfers an electron and then a carbanion is formed from the alkyl radical. Then another alkyl halide is attacked by the carbanion and this involves an SN2 mechanism. Finally this gives rise to the alkanes. Now the reaction can happen between the methyl iodide molecules, the ethyl iodide molecules as well as the between the methyl iodide and ethyl iodide. As the sodium atoms donate the electrons hence both the options are formed and statement –II is a correct explanation to the statement-I. The mechanism has been provided below for a better understanding of the reaction.
Note:
In the presence of dry ether, two sodium metal atoms react with the halide group of the alkyl halide to form sodium halide, among which the general groups include bromide and iodide.
The Wurtz reaction was named after Chalres Adolphe Wurtz and this was invented for the coupling of organic molecules to form larger molecules in organic and organometallic chemistry. The reaction proceeds through a nucleophilic substitution pathway and leads to the carbon-carbon bond formation. The sodium metal being a strong reducing agent transfers its electron to the halogen atom to form the metal halide and the alkyl radical which accepts an electron from another metal atom to form the alkyl anion. The nucleophilic alkyl anion thus attacks another alkyl halide from the backside leading to the formation of the alkane and the halide anion leaves.
