Question
Question: Statement-I: \(p{K_{a1}}\) of fumaric acid is more than maleic acid. Because Statement-II: Conju...
Statement-I: pKa1 of fumaric acid is more than maleic acid.
Because
Statement-II: Conjugate base of fumaric acid is stabilized by intramolecular H-bonding.
A.Statement-I is True. Statement-II is a correct explanation for Statement-I
B.Statement-I is True. Statement-II is true; Statement-II is NOT a correct explanation for Statement-I
C.Statement-I is True, Statement-II is False.
D.Statement-I is False. Statement-II is True.
Solution
Fumaric acid is trans-butene-dioic acid and maleic acid is cis-butene-dioic acid. The pKa value is a method used to indicate the strength of an acid. pKa is the negative log of the acid dissociation constant. A lower pKa value indicates a stronger acid.
pKa=−log[Ka]
Complete step by step answer:
Fumaric acid is also known as trans- butene-dioic acid.
Trans means both the −COOH (carboxyl group attached at the opposite sides of the carbon chain due to which its conjugate base cannot be stabilized by intramolecular hydrogen bonding. The anion formed in fumaric acid is less stable and it's Ka (dissociation constant) will be less which means it is less acidic.
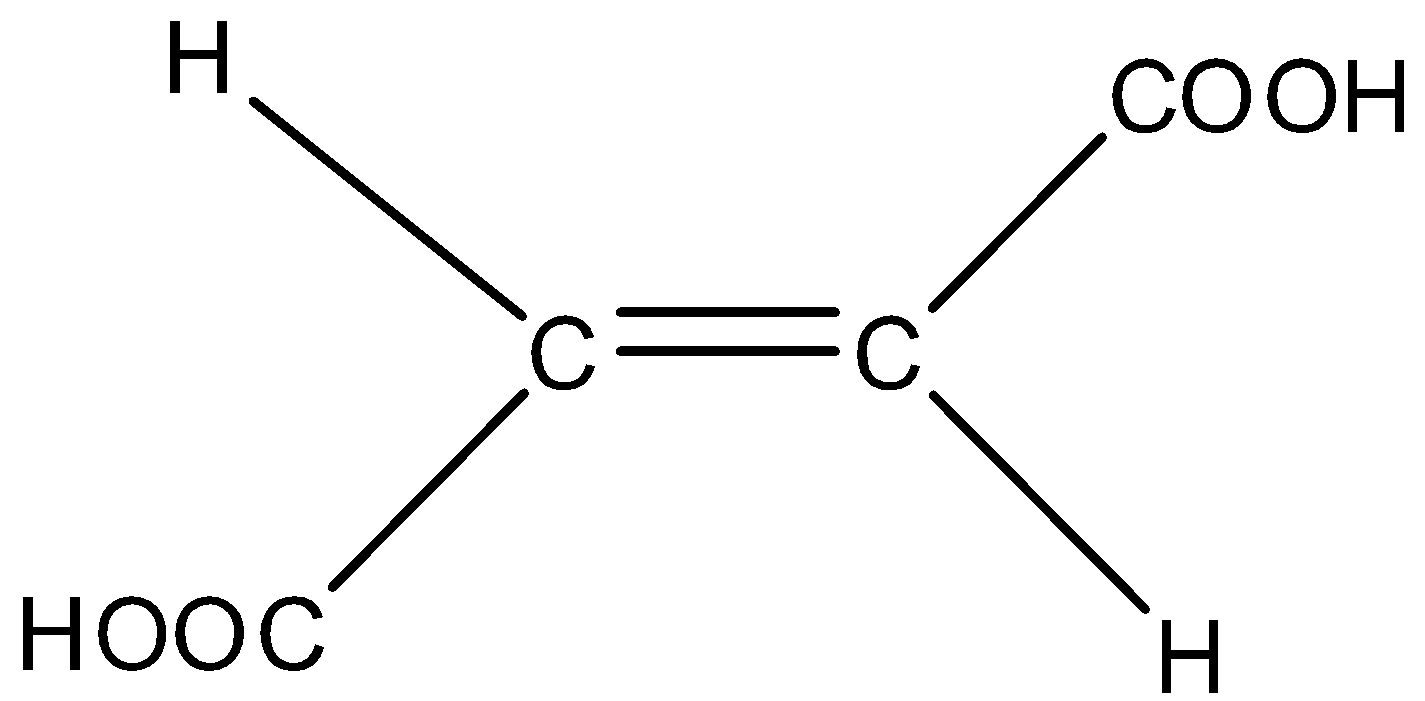
Maleic acid is also known as cis –butene-dioic acid.
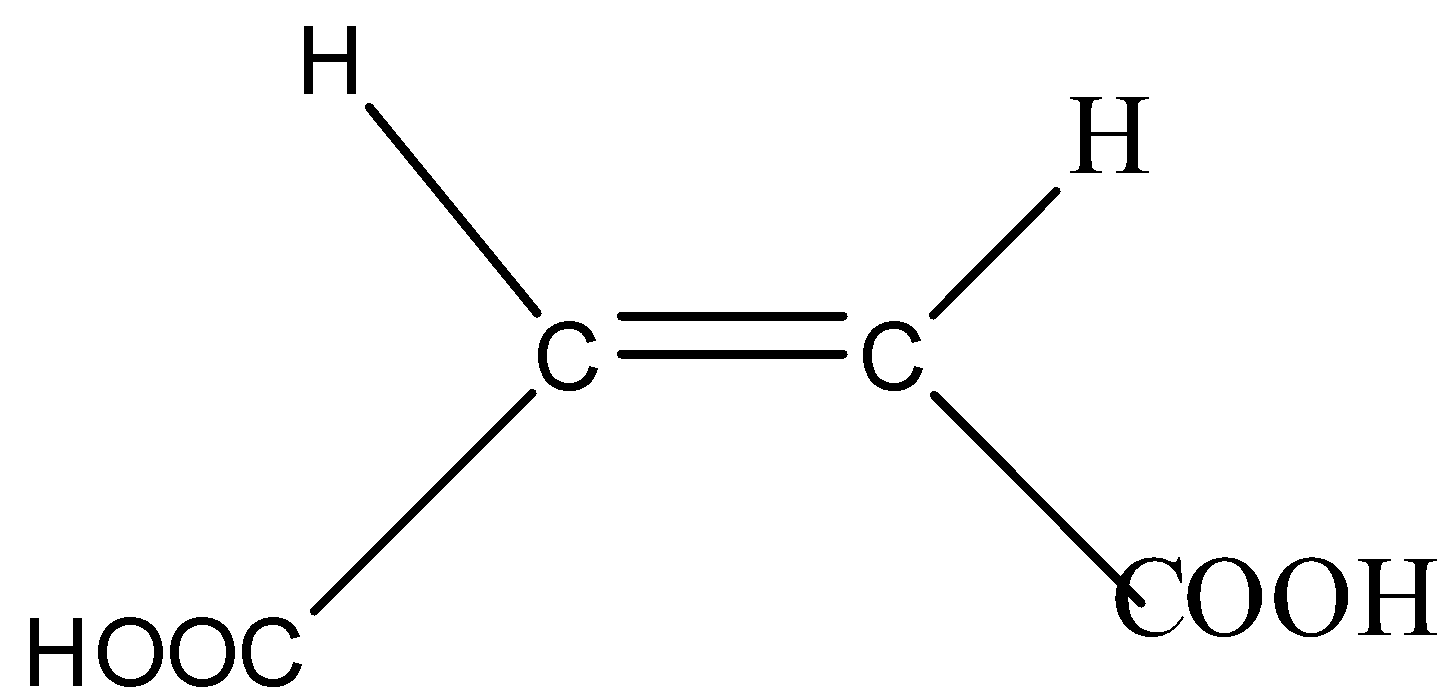
Cis means both the −COOH (carboxylic group) attached at the same side of the carbon chain due to which the anion of maleic acid can be stabilized by intramolecular hydrogen bonding.
The anion formed in maleic acid is more stable due to hydrogen bonding and its Ka (dissociation constant) will be more which means it is less acidic. The conjugate base of maleic acid is stabilized by hydrogen bonding.
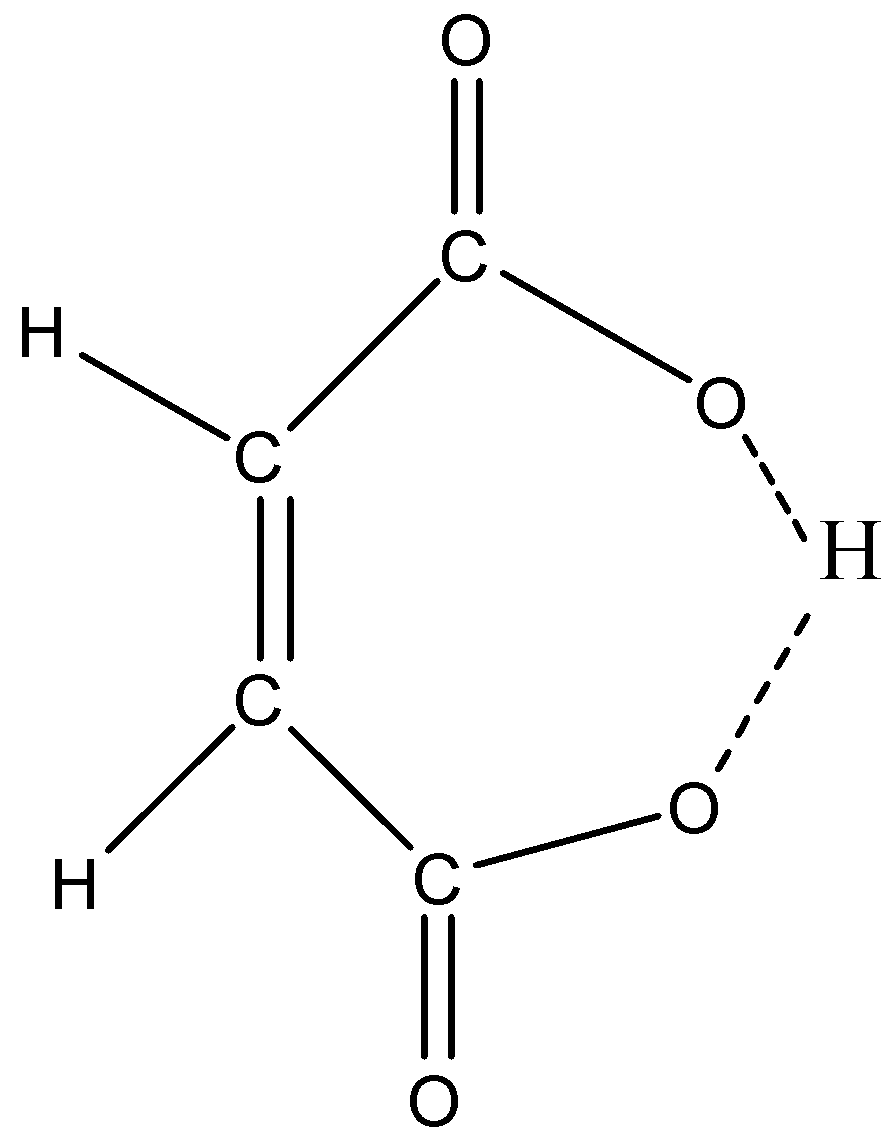
For an acid whose Ka value will be more will have less pKa value.
So we can say that pKa1 of fumaric acid is more than maleic acid, but the conjugate base of fumaric acid is not stabilized by hydrogen bonding.
Hence, the correct option is (C).
Note:
Uses of Fumaric acid
It is used in the manufacture of polyester resins and polyhedric alcohols and as a mordant for dyes in the industries.
It has been used as a food acidulant since 1946.
Uses of Maleic acid
Maleic anhydride and maleic acid are the important raw materials used in the manufacture of phthalic-type alkyd and polyester resins, surface coatings, lubricant additives, plasticizers, copolymers, and agricultural chemicals.
