Question
Question: Statement I: \({{Buta - 1,3 - diene}}\) is less stable than \({{penta - 1,4 - diene}}\). Statement...
Statement I: Buta−1,3−diene is less stable than penta−1,4−diene.
Statement II: Buta−1,3−diene has a greater number of resonating structures and the delocalized electron cloud.
A.Statement- I is true, Statement -II is true; Statement II is a correct explanation for Statement-I
B.Statement- I is true, Statement -II is true; Statement II is not a correct explanation for Statement-I
C.Statement- I is true, Statement -II is false
D.Statement- I is false, Statement -II is true
Solution
The compound having lone pairs, double or triple bond have a maximum chance of having resonance. The compound having a large number of resonating structures is more stable. The conjugate double bond makes the compound more stable as seen in hyperconjugation.
Complete step by step answer:
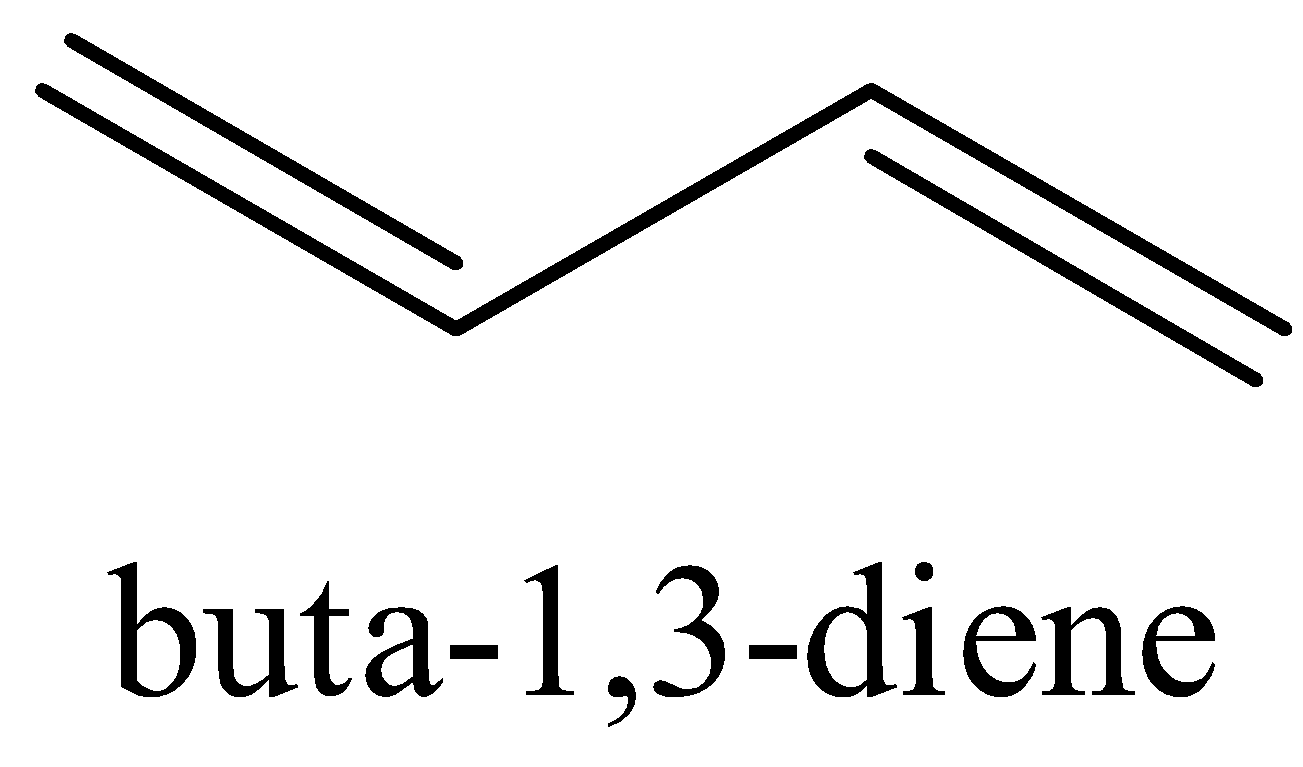
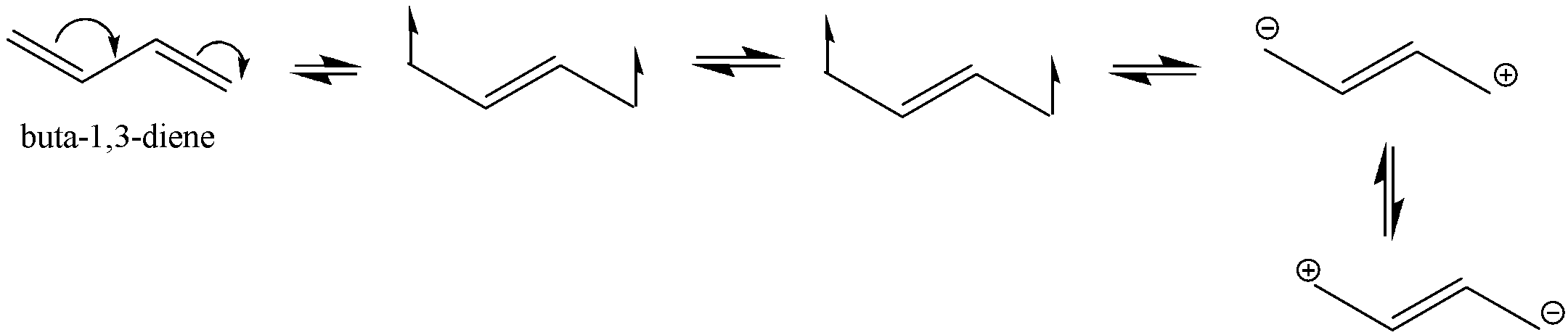
The structure Buta−1,3−diene is shown below with its all resonating structures.
We can see that there are conjugated double bonds in Buta−1,3−diene which means that the single bond and double bond alternates.
Thus, it leads to resonance structures. It enables the electrons to be delocalized to the whole system. We know that a molecule with more resonance structures is more stable.
The structure penta−1,4−diene is shown below
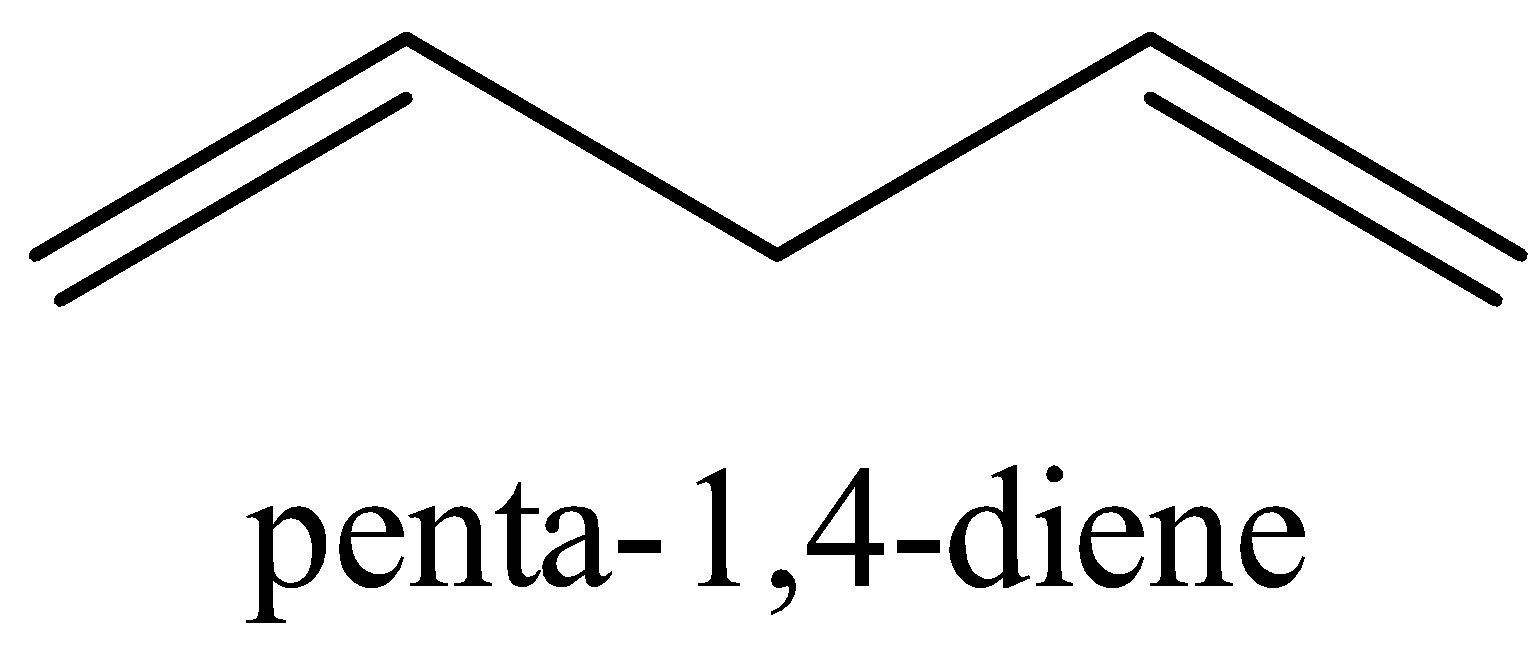
We could see that there are no conjugated double bonds in penta−1,4−diene.
Thus, it does not have resonance structures.
As we can see that Buta−1,3−diene has 4 resonating structures and penta−1,4−diene does not show resonance.
So we can conclude that Buta−1,3−diene is more stable since it shows resonance. The more the resonating structures, the more is the stability.
Hence the statement I is false and the statement II is true.
Hence, the correct option is D.
Additional information: The stability of carbocation is as follows:- tertiary>secondary>primary. The compound having less energy is more stable. The compound with high energy is less stable.
Note:
To find the stability between two compounds the first focus should be to find the number of resonating structures shown by the compounds. If the compound is having more conjugate bonds then it is more stable. A Conjugate system is a system of bonds having p orbitals with delocalized electrons in a molecule.
