Question
Question: Statement I: Alpha particles are the most penetrating radioactive particle. Statement II: Alpha pa...
Statement I: Alpha particles are the most penetrating radioactive particle.
Statement II: Alpha particles are the smallest of the radioactive particles.
A) Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I.
B) Both Statement I and Statement II are correct but Statement II is not the correct explanation of Statement I.
C) Statement I is correct, Statement II is incorrect.
D) Both Statement I and Statement II are incorrect.
Solution
An alpha particle is identical to a helium atom that has been stripped of its two electrons; thus, an alpha particle contains two protons and two neutrons. Because an alpha particle has no electrons to balance the positive charge of the two protons, it has a charge of +2.
Complete step by step answer:
-When a radiation particle interacts with atoms, the interaction can cause the atom to lose electrons and thus become ionized. The greater the likelihood that damage will occur by an interaction is the ionizing power of the radiation.
-Alpha particles have 2 neutrons and 2 protons. Hence, Alpha particles are not the smallest of the radioactive particles. They have a very very high mass. It is doubly ionised helium ion and is represented as:
24He
-Because of the high mass of the alpha particles, they have a very low penetrating power. Except for long range alpha particles, which have somewhat larger penetration power.
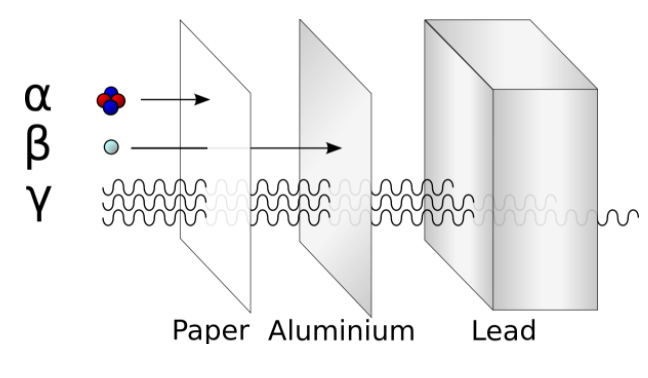
-Comparing only the three common types of ionizing radiation, alpha particles have the greatest mass. Alpha particles have approximately four times the mass of a proton or neutron and approximately 8,000 times the mass of a beta particle.
- Because of the large mass of the alpha particle, it has the highest ionizing power and the greatest ability to damage tissue. That same large size of alpha particles, however, makes them less able to penetrate matter.
-They collide with molecules very quickly when striking matter, add two electrons, and become a harmless helium atom. Alpha particles have the least penetrating power and can be stopped by a thick sheet of paper or even a layer of clothes.
So, the correct answer is “Option D”.
Note: They can cause multiple ionisations within a very small distance. This gives them the energy to do much more biological damage in that energy. Alpha particles can't penetrate the normal layer of dead cells on the outside of our skin but can damage the cornea of the eye.
