Question
Question: Statement: Aluminium chloride \(\text{ AlC}{{\text{l}}_{\text{3}}}\text{ }\) is a Lewis acid because...
Statement: Aluminium chloride AlCl3 is a Lewis acid because it can donate the electron.
If a given statement is true enter 1 if false enter 0.
Solution
Lewis suggested the acid-base concept. According to which the Lewis base is a chemical species that can donate the electron pair and Lewis base is a chemical species that can easily accept the electron pair. AlCl3 has an empty orbital and accommodates the electron pair in an empty orbital.
Complete answer:
Lewis acid is an ion or a molecule that accepts the nonbonding valence electrons in its empty orbital. Lewis base is an ion or molecule which can donate an electron pair.
Aluminium trichloride AlCl3 is an electron-deficient species.it has three electrons in the valence shell. The electronic configuration of aluminium is Al = [Ne]3s23p13d0
In AlCl3 aluminium loses its three valence shell electrons. Two electrons from the 3s orbitals and one electron from the 3p orbital. When bonded with chlorine Al3+ has empty 3s, 3p, and 3d orbitals. These empty orbitals are used to accommodate the nonbonding pair of the electron which are donated by the Lewis base.
Let’s consider a reaction of Al3+ the water molecule. The Al3+ ion is an electron-deficient species and bonds three chloride molecules. The bonding between the aluminium and chloride is as shown below,
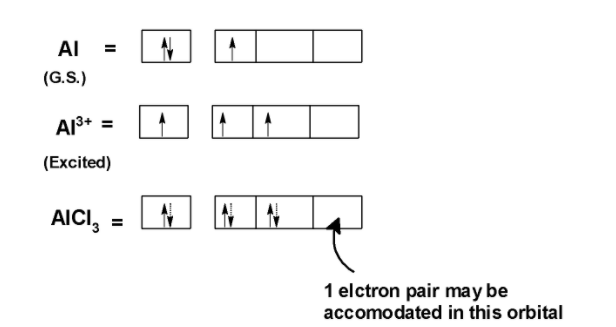
One orbital of the AlCl3 is empty. This orbit can accommodate the nonbonding electron pair of electrons. Thus AlCl3 acts as a Lewis acid because it can accept an electron.
Thus the given statement is false.
Hence, ‘0’ is the correct answer.
Note: Lewis acid is used as a catalyst in Friedel craft reaction. AlCl3 accepts the lone pair from chloride ion and form the AlCl4− ion. This forms a carbonium ion which further acts as a strong Lewis acid. Thus Friedel craft acylation and alkylation reactions are facilitated by AlCl3 the catalyst.
