Question
Question: **STATEMENT-1** : The plot of atomic number (y-axis) versus number of neutrons (x-axis) for stable n...
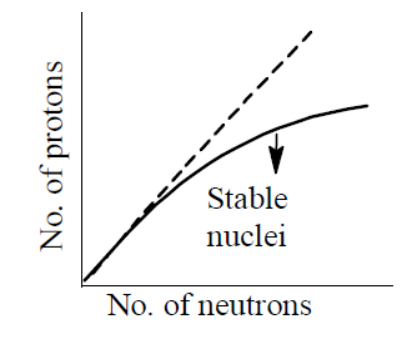
STATEMENT-1 : The plot of atomic number (y-axis) versus number of neutrons (x-axis) for stable nuclei shows a curvature towards x-axis from the line of 45o slope as the atomic number is increased.
STATEMENT-2: Proton-proton electrostatic repulsions begin to overcome attractive forces involving protons and neutrons and neutrons in heavier nuclides.
A) STATEMENT-1 is True; STATEMENT-2 is True; STATEMENT-2 is the correct explanation for STATEMENT-1.
B) STATEMENT-1 is True; STATEMENT-2 is True; STATEMENT-2 is NOT a correct explanation for STATEMENT-1.
C) STATEMENT-1 is True; STATEMENT-2 is False.
D) STATEMENT-1 is False; STATEMENT-2 is True.
Solution
Atomic number indicates numbers of protons present in the nucleus or number of electrons outside the nucleus. The mass number indicates the total number of protons and neutrons present in the nucleus. The number of neutrons can be calculated by taking the difference between mass number and atomic number.
Complete step by step answer:
The fundamental particles of an atom are election, proton and neutron. Protons and neutrons are present in the nucleus and electrons are revolving around the nucleus.
With advantage in the atomic number, the proton-proton repulsion begins to overaffect attractive forces between proton and neutrons in heavier nuclides.
Hence, the plot of atomic number (y-axis) vs. number of neutrons (x-axis) for stable nuclei shows a curvature towards x-axis from the line of 45o slope.
Thus, Both STATEMENT-1 and STATEMENT-2are correct and STATEMENT-2 is the correct explanation of STATEMENT-1.
Additional Information: The mass of an atom is concentrated at the nucleus because the mass of atom is due to proton and neutron and they are present in the nucleus. Mass of the electron is negligible; it is revolving around the nucleus. An atom is neutral as a whole therefore number protons (positive charge) and number of electrons (negative charge) are the same.
Note: Mass number indicates sum of protons and neutrons present in the nucleus which is also called nucleons. Atomic number indicates the number of electrons or number of protons. So, the number of neutrons can be calculated by taking the difference of a mass number and atomic number.
