Question
Question: Statement 1: The molecule \(S{O_2}\) has a net dipole. Statement 2: Oxygen has a higher electroneg...
Statement 1: The molecule SO2 has a net dipole.
Statement 2: Oxygen has a higher electronegativity than sulfur.
A.Both Statement 1 and statement 2 are correct and Statement 2 is the correct explanation of Statement 1.
B.Both Statement 1 and statement 2 are correct, but Statement 2 is NOT the correct explanation of Statement 1.
C.Statement 1 is correct, but Statement 2 is not correct.
D.Statement 1 is not correct, but Statement 2 is correct.
Solution
For answering this question we need to understand the concept of polarity of bonds. A dipole moment is a physical quantity that is used for the measurement of polarity of covalent bonds. We can define dipole moment as the product of the magnitude of the charge and the distance between the centers of positive and negative charges.
Complete step by step answer:
So, here in the SO2 molecule let’s first check the dipole moment between the atoms of a molecule.
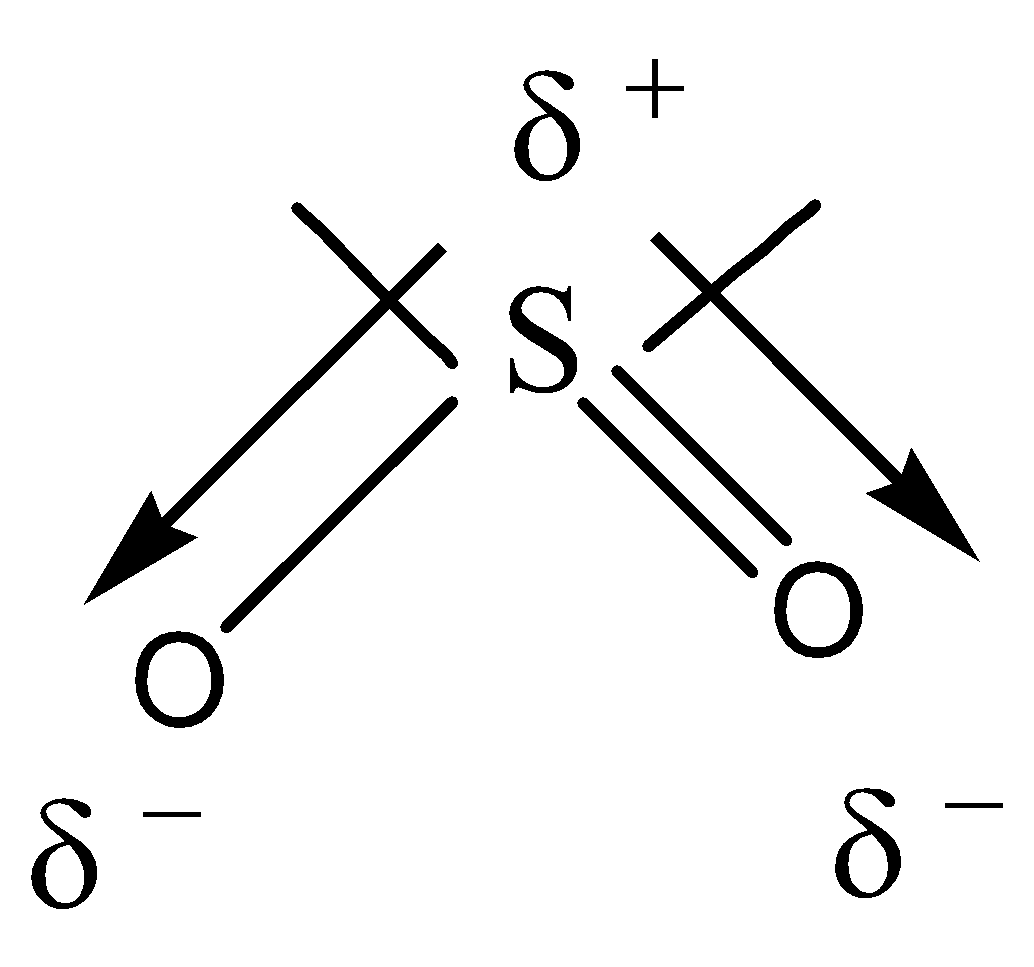
As we know that in the molecular structure dipole moment can be described as the polarity of the molecule. By looking at the structure of the SO2 molecule it is evident that it has a bent structure.
The bent structure of the SO2 due to the electrons stay as far apart from each other as possible in the molecule. Here, the central atom of sulfur S contains 6 valence electrons like Oxygen O. Also, we are aware that a multiple or a double bond has higher electron density than a single bond. With reference to the concept of dipole moment, we get to know that the Oxygen atom has a stronger pull on the electron in the bond between S and O, which can be seem more easily using vectors.
Thus, Both Statement 1 and statement 2 are correct, but Statement 2 is NOT the correct explanation of Statement 1 (option B). As electronegativity of an atom cannot be measured with reference to the dipole moment of a molecule.
∴ Option B is the correct answer.
Note:
Thus, we also need to keep in mind that the Dipole moment helps us to find out the difference between polar and nonpolar molecules. Also, it helps to determine the ionic character of a molecule. Thus, it can be used as an important tool to decide the geometry of a molecule.
