Question
Question: State True or False The graph between PV vs P at constant temperature is linear parallel to the pr...
State True or False
The graph between PV vs P at constant temperature is linear parallel to the pressure axis.
A. True
B. False
Solution
Hint: “When temperature is constant, then the product of pressure and volume is constant. This relationship is known as Boyle's law or Mariotte's law”. At constant temperature the process is called as isothermal.
Complete step by step answer:
- We know that the Ideal gas equation is PV= nRT where P = Pressure V = Volume n = Number of moles of gas R = Universal, gas constant. T = Temperature of the gas
Subsequently if temperature is kept constant the RHS of the equation (nRT) is also constant.
Therefore PV= Constant
If we are going to draw a graph, PV against P, it will be a straight line parallel to the P axis.
So, the given statement is true.
So, the correct option is A.
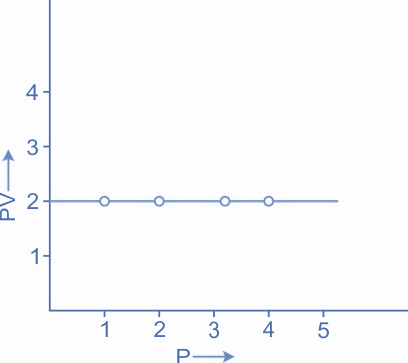
The above graph explains the same concept, a graph, PV against P it will be a straight line parallel to the P axis.
Note: Don’t be confused with the words isothermal and isobaric process. Both are different.
Isothermal process: Isothermal process is a thermodynamic process in which the temperature is constant
Isobaric process: Isobaric process is a thermodynamic process in which the pressure is constant.
If “PV = constant”, then it is called as Boyle’s law, here temperature is constant.
