Question
Question: State the meaning of functional group in a carbon compound. Write the functional group present in ...
State the meaning of functional group in a carbon compound. Write the functional group present in
(a) ethanol
(b) ethanoic acid
And draw their structures.
Solution
In organic chemistry, functional groups are specific substituents or moieties within molecules that may be responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of.
Complete step by step answer:
We have been asked about the functional group,
So, for that:
- Functional groups are specific groupings of atoms within molecules that have their own characteristic properties, regardless of the other atoms present in a molecule. Common examples are alcohols, amines, carboxylic acids, ketones, and ethers.
| Organic compound | Functional group |
|---|---|
| Haloalkanes | Halide-X(Cl, F, Br, I) |
| Alcohols | Hydroxyl-OH |
| Aldehydes | Hydroxyl-CHO |
| Carboxylic acid | Carboxyl-COOH |
| Ketones | Keto, 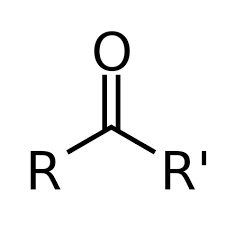 |
| Ethers | Ethers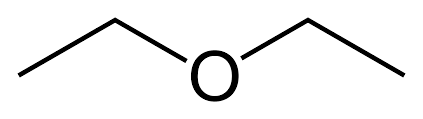 |
We have been given ethanol whose structure is:
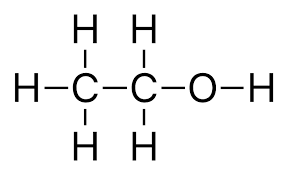
In this the functional group is hydroxyl-OH,
So, it belongs to the alcohol group.
Then we have, ethanoic acid whose structure is:
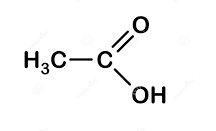
- In this the functional group is carboxyl-COOH,
So, it belongs to the carboxylic acid group.
So, we can say that the functional group present in ethanol is alcohol and ethanoic acid is carboxylic acid.
Note: CARBOXYLIC ACIDS (highest priority among carbon-containing functional groups).
-CARBOXYLIC ACID DERIVATIVES.
-OTHER GROUPS CONTAINING OXYGEN OR NITROGEN.
-ALKENES AND ALKYNES.
Note: substances containing double and triple bonds are called alkenynes. (notice that the name ends in yne).
