Question
Question: State the electronic configuration for Nitrogen \[\left[ \mathbf{p}=\mathbf{7},\text{ }\mathbf{n}=\m...
State the electronic configuration for Nitrogen [p=7, n=7]
Solution
As we know, atomic number is total number of a proton present in a nucleus of atom as well as atomic mass number is total number of a proton along with neutron. Nitrogen differs from other elements of the group 15 due to its high electronegative character small size as well as high ionization enthalpy. Nitrogen could form multiple bonds with itself as well as other elements. It forms pp multiple bonds.
Complete step-by-step answer: The atomic number of nitrogen is 7 . This is the number of protons in a nuclei of nitrogen atoms. A neutral atom has the same number of electrons as protons. So electron configuration will include 7 electron placed into appropriate s along with p orbitals in ground state, state of a lowest energy. The full electron configurations for nitrogen is 1s22s22p3
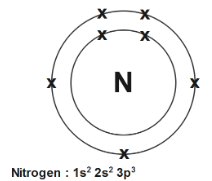
So the nitrogen has an atomic number 7 as well as mass number 7+7=14 , K shell will place 2 electrons as well as rest will be accommodated into L shell. Therefore configuration is 2, 5 just adding power of configuration also we know that s hold 2 electron so the electron at 2s2 will be at second subshell since second subshell p holds up to 6 electrons so 2 electrons from 2s2 and 3p3 which sums up and gives, 5 electron in p−orbital
Therefore, the electronic configuration of Nitrogen is 2, 5.
Note: Note that atomic number of nitrogen is 7 . This is the number of protons in the nuclei of nitrogen atoms. A neutral atom has the same number of electrons as protons. So the electronic configurations will include 7 electron placed into appropriate s as well as p orbital in ground state/state of lowest energy.
