Question
Question: State reasons for the following: \( S{F_6} \) is kinetically inert substance...
State reasons for the following:
SF6 is kinetically inert substance
Solution
Sulphur hexafluoride ( SF6 ) is an inorganic, colourless, odourless, non-inflammable, non-toxic and hypervalent molecule. It is usually used as an electrical insulator and arc suppressant. It has a density higher than air and is usually transported as liquefied compressed gas.
Complete step by step solution:
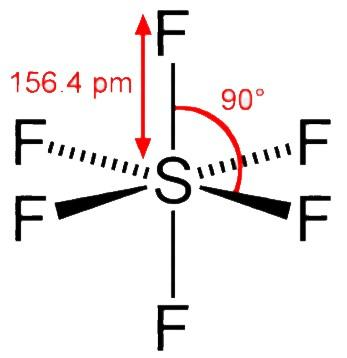
Sulphur hexafluoride ( SF6 ) has an octahedral geometry where the sulphur atom is being surrounded by six fluorine atoms. Sulphur hexafluoride ( SF6 ) is usually prepared by exposing sulphur ( S8 ) to fluorine ( F2 ). It is an inorganic non - polar gas. SF6 is sp3d2 hybridised and during its formation the central sulphur atom is in its ground state having 3s23p4 configuration. It consists of six sp3d2 hybridised orbitals that are projected towards six corners of a regular octahedron.
In SF6 the central sulphur atom is sterically surrounded by the protection of six fluorine ( F ) atoms where the F−S−F bonds are slated to be at an angle of 90∘ . The fluorine atom surrounding the central sulphur atom protects the sulphur atom without allowing the water molecules to get in. Also, fluorine doesn’t have vacant d− orbitals to accept the electron which is being donated by H2O .
Thus SF6 stays to be kinetically inert.
Note:
SF6 is a type of greenhouse gas which is colourless, odourless, non-toxic and non – flammable. It is inert in nature in the troposphere and stratosphere. SF6 is commonly used as a high voltage dielectric in high voltage supplies.
