Question
Question: State Kirchhoff’s law of radiation and prove it theoretically....
State Kirchhoff’s law of radiation and prove it theoretically.
Solution
Hint: Kirchhoff’s law of thermal radiation states that the emissive power to the coefficient of absorption is constant for all the substances at a given temperature. We can say that at a given temperature, the emissivity of a substance is equal to the coefficient of absorption.
Formula used:
aE=constant =Eb
Complete step by step answer:
In heat transfer, Kirchhoff’s thermal radiation law refers to the wavelength-specific radiative emission and absorption by a body in thermodynamic equilibrium. Kirchhoff’s law states that: For a body made up of any arbitrary material, the emitting and the absorbing thermal electromagnetic radiation, the ratio of its emissive power to its coefficient of absorption is equal to a universal function. That universal function describes the emissive power of a perfect black body.
Explanation of Kirchhoff’s thermal radiation law:
If E is the emissive power of a substance and a is the coefficient of absorption, then by Kirchhoff’s law of radiation,
aE=constant =Eb
Or we can say,a=e
Theoretical proof of Kirchhoff’s law of thermal radiation:
We will consider two bodies A and B being suspended in a constant temperature enclosure. A is a normal material body and B is a perfectly black body. After some interval of time both A and B will attain the same temperature as that of the enclosure. Prevost heat exchange theory states that every body will emit and absorb thermal radiations.
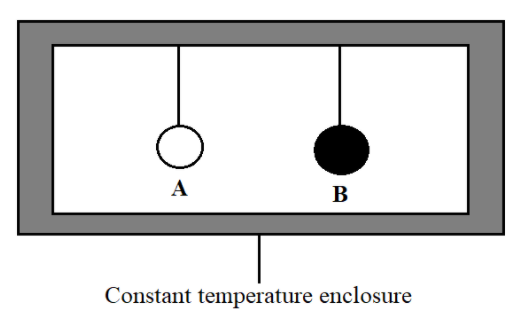
Let E be the emissive power of A and a be its coefficient of absorption. Let Eb be the emissive power of B. Let Q be the radiant heat incident per unit time area of each body.
Heat absorbed by body A per unit time per unit area = aQ
Heat emitted by body A per unit time per unit area = E
As the temperature remains constant, heat absorbed will be equal to the heat emitted
E=aQ
Perfectly black body will absorb the entire heat incident on it
Heat absorbed by body B per unit time per unit area = Q
Heat emitted by body B per unit time per unit area = Eb
As the temperature remains constant, heat absorbed will be equal to the heat emitted
Eb=Q
From above equations, we get
EbE=QaQEbE=a
But, EbE=e=coefficient of emission
Therefore, a=e
Thus, the coefficient of emission is equal to the coefficient of absorption. This proves Kirchhoff’s thermal radiation law theoretically.
Note: Students should keep in mind that Emissivity is a measure of how strongly a body interacts with thermal radiation. Always remember, a high emissivity of material comes together with a high absorptance. Emissivity of a black body is 1.
